What are Addition Compounds?
When two or more stable compounds are combined together in stoichiometric proportions, molecular or Addition compounds are formed. Example-
| K2SO4 + Al2(SO4)3 + 24 H2O ————-> K2SO4 . Al2(SO4)3 . 24 H2O (Potash Alum) CuSO4 + 4 NH3 ————> CuSO4 . 4 NH3 (Tetraammine Copper Sulfate) |
These addition compounds are of two types-
(I) Double Salts- These addition compounds are stable in the solid state, but dissociate into their constituents when dissolved in water or melted. For example- an aqueous solution of Potash Alum shows the properties of K+, Al+3 and SO4-2 ions.
(II) Coordination Compounds- These addition compounds retain their identity in the solid as well as in the dissolved state and the individual properties of the constituents are usually lost in these compounds. Example blue coloured solution of Tetraamine copper sulfate does not show the presence of Cu+2 ions but instead contains Cupro ammonium ions [Cu(NH3)4]+2. These compounds contain a central metal atom or ion surrounded by a suitable number of ions or neutral molecules with the help of coordinate bonds. Thus, the formation of coordinate or complex compounds involves two things-
An acceptor- also known as a central metal atom or ion which is usually a metal to which one or more neutral molecules or anions are attached by donating a pair of electrons. For example in [Fe(CN)6]4-, Fe2+ ion is the acceptor.
A Donor- which is an electron rich atom or molecule which can donate a pair of electrons and is also known as “Ligand” i.e. the neutral molecules or negatively charged ions (anions) that surround the metal ion in a complex compound is known as “Ligands”. Example- in [Fe(CN)6]4-, CN– ion is the Ligand. Other examples of Ligands are-
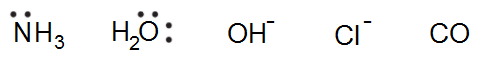
The atom in the Ligand which forms a coordinate bond with the central metal atom is known as the coordinating atom or Donor atom or Donor site of the Ligand. “Ligands with more than one donor site are known as “Polydentate or Multidentate Ligands“.









Comments (No)