Table of Contents
What is Coulomb’s Law?
In 1785 Coulomb deducted a law for the forces of attraction and repulsion between charged bodies. He formulated the law on the basis of direct measurement of the electrostatic force between the charged bodies.
Coulomb used a delicate torsional balance for the measurement of force between two charged bodies. In one set of measurements, he changed the distance between the charged bodies and found that the force between the two charged bodies followed an inverse square law. At the time Coulomb performed these investigations, no method of measuring the quantity of charge was available. However, he decreased the charge on the charged bodies in the desired proportions by sharing it with other similar but uncharged bodies. His experimental results clearly showed that at a given separation, the force between two charged bodies depends directly on the product of their individual charges.
Hence, according to this law, the force of attraction/repulsion between the two charges at rest is directly proportional to the product of the two charges and inversely proportional to the square of the distance between them.
Explanation of Coulomb’s Law in Scalar Form:
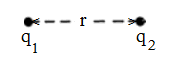
If q1 and q2 be the two charges at rest and ‘r’ be the distance between them. Then,
| F ∝ q1 q2 ………….(i) F ∝ 1/r2 ……………(ii) Combine (i) and (ii), we have- F ∝ q1 q2/r2 ⇒ F = k q1 q2/r2 |
Where ‘k’ is constant of proportionality. It is called Coloumb’s constant. In S.I. UNits k = 1/4πε0 , where ‘ε0‘ is called as permittivity of free space. Also the value of ε0 = 8.85 x 10-12 C2/N-m2.
| ⇒ F = 1/4πε0 x q1 q2/r2 …………….(I) ⇒ F = 1/4 x3.14 x 8.85 x 10-12 x q1 q2/r2 ⇒ F = 9 x 109 x q1 q2/r2 …………….(II) |
It should be noted that Equations (I) and (II) are the expressions for Coloumb’s Law, when the two charges are placed in free space.
If the two charges are placed in medium and let ‘ε’ be the permittivity of the medium, then Coulomb’s Law can be written as-
| Fm = 1/4πε x q1 q2/r2 …………..(III) But ε = ε0k ⇒ Fm = 1/4πε0k x q1 q2/r2 ……….(IV) |
Importance of Coulomb’s Law:
It enables us to calculate electric force between two point charges at rest.
Limitations of Coulomb’s Law:
(I) It is applicable in case of point charges only. It is not applicable when charged bodies are spheres etc.
(II) It does not apply, when charges are in motion.
Important Note:
If q1 q2 > 0 i.e. both the charges are positive or negative, then the two charges repel each other and if q1 q2 < 0 i.e. if one charge is positive and other charge is negative, then two charges attract each other.
Important Features of Coulomb’s Law:
(1) It is an Experimental Law.
(2) Coulomb’s Law is valid for stationary point charges.
(3) Coulomb’s force between two point charges is mutual force.
(4) The Coulomb’s force depends upon these three factors i.e.
- Magnitude of charges.
- Distance between charges.
- Medium surrounding the charges.
(5) For unlike charges, Coulomb’s force is attractive and for like charges Coulomb’s force is repulsive.
(6) Coulomb’s force is not affected by the presence of other neighbouring charges.
(7) Coulomb’s force is valid for distance from 10-15 m to several km.
(8) Coulomb’s forces are much stronger than gravitational forces.
(9) Coulomb’s force is a central force.








Comments (No)