Crystal Field Theory:
This theory was developed by Hans Bethe (1929) and John Van Vleck (1932). The main postulates of this theory are-
(1) According to this theory there exist an electrostatic force of attraction between the metal ion and ligand i.e. this theory consider the ionic bond between metal ion and ligand.
(2) The interactions between metal ion and ligand are similar to those between the ions in a crystal that is why this theory is known as crystal field theory (CFT).
(3) It considers ligands as negative point charge and central metal ion as a positive point charge.
(4) Ligand creates a negative field around the central metal ion. If the ligand is negatively charged then a negative field is created by the charge present on it. But, if the ligand is neutral, then the negative pole of that ligand will create the negative field around the central metal ion.
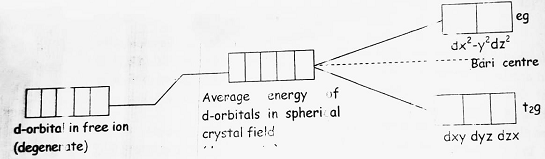
(5) If ligand created a spherically symmetrical field around the central metal ion then the d-orbital present will remain degenerate. But, if the field is unsymmetrical then the degeneracy of d-orbital will split down into two groups viz t2g (triply degenerate) and eg (doubly degenerate).
(6) In octahedral complexes, the ligand field approaches towards the central metal ion from the axis therefore, orbitals in which electron cloud is present on the axis will feel more repulsion than the orbitals where the electron cloud is present on the axis. This will result in the splitting of d-orbitals as-
CFSEoh = – 0.4(t2g)n + 0.6(eg)n , Where n = number of electrons present
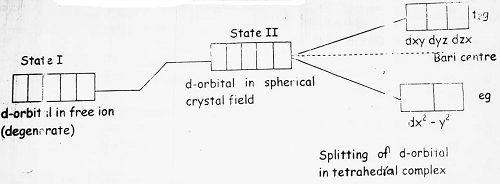
(7) In tetrahedral complexes, the ligand field approaches towards the central metal ion between the axis, therefore dxy, dyz and dzx will feel more repulsions than dx2 – y2 and dz2. Splitting can be shown as-
CFSEth = – 0.6(eg)n + 0.4(t2g)n , Where n = number of electrons present
Applications of Crystal Field Theory:
(1) It helps us to calculate crystal field stabilization energy. It is usually represented by Δ0 or Δt. Example- CFSE for d4 ion in octahedral complexes will be-
(a) d4 (low spin)
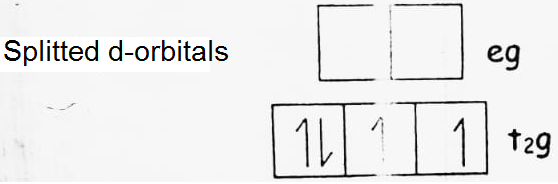
CFSE = -0.4 x 4 + 0.6 x 0 = -1.6 Δ0
(b) d4 (high spin)
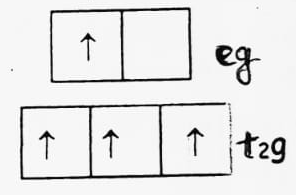
CFSE = -0.4 x 3 + 0.6 x 1 = -1.2 + 0.6 = -0.6 Δ0
Similarly, CFSE for tetrahedral complexes can be calculated by using the formula-
(CFSE)th = -0.6(eg)n + 0.4(t2g)n
(2) It helps us to explain the formation of low spin and high spin complexes. Strong field ligands split d-orbital up to large extent. Therefore pairing of electrons takes place and the complex is low spin.
(3) It also explains the colour of the complexes. All these complexes, where the d-orbital contain 1 to 9 electrons will undergo d-d transition. Therefore, they are coloured. Example- [Sc(H2O)6]2+, Ti2+ ion, VO2+, Cr+1, Mn+2, Co+3 and Ni+2.
Note- KMnO4 and K2Cr2O7 are coloured because of charge transfer spectra.








Comments (No)