Table of Contents
Heating Effect of Current:
Whenever the electric current is passed through a conductor, it becomes hot after some time. This is called the heating effect of current or Joule’s heating effect. The function of an electric bulb, electric press, geyser, immersion rod, etc. is based on Joule’s heating effect.
Statement of Joule’s Law:
It states that the amount of heat produced (Q) in a conductor is directly proportional to-
- Square of current (I) flowing through the conductor.
- Resistance (R) of the conductor.
- The time (t) for which current is passed.
| i.e. Q ∝ I2RT ⇒ Q = K x I2RT In S.I.units, K = 1, ⇒ Q = I2RT Joules ⇒ Q = I2RT/4.18 Calories |
Expression for Joule’s Law:
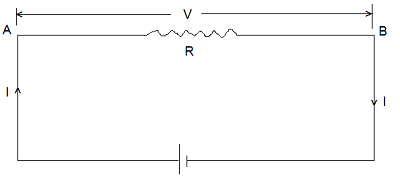
Consider a conductor AB of resistance R. Let ‘V‘ be the potential difference applied across the ends AB. Let ‘I‘ be the current flowing through conductor AB and let ‘t‘ be the time for which the current is flowing. Therefore, the charge flowing from A to B in time ‘t‘ is given by-
| q = I x t …………(i) |
Work done in carrying a charge ‘q’ from A to B is given by-
| W = q x V Using equation (i), we get- W = V x I x t |
The energy equivalent to this is expended in the form of heat dissipation. Taking ‘V’ in volt, ‘I’ in amperes and ‘t’ in second this heat energy dissipation (Q) is obtained in joule. We may write-
| Q = V x I x t ……………(ii) Other equivalent forms of equation (ii) are written, by using Ohm’s Law (V = I x R) Q = V2/R x t Joule Q = I2RT Joule Q = I2RT/4.18 Calories |
Cause of Heating Effect of Current:
When a battery is connected to the ends of a conductor, an electric field is set up in the conductor. The free electrons of the conductor get accelerated in a direction opposite to the direction of the electric field. They suffer frequent collisions with the positive ions and atoms of the conductor. The average kinetic energy of vibration of the ions and atoms is increased. This will raise the temperature of the conductor. Thus, the energy supplied by the source of emf gets converted into heat energy.







Comments (No)