Table of Contents
What is Fuel Cell?
The galvanic cell in which the energy of the combustion of fuels is directly converted into electrical energy is called the fuel cell. The most common fuel cell is the Hydrogen-Oxygen Fuel Cell or H2-O2 Fuel Cell. Fuel cells are primary cells and reactants must be continuously supplied to them.
Construction of H2-O2 Fuel Cell:
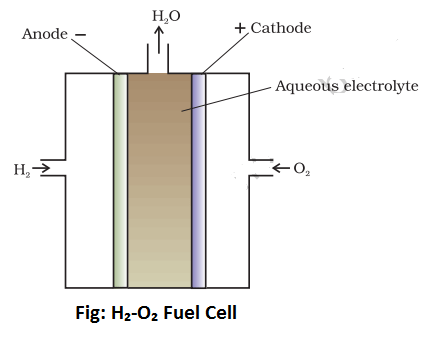
The body of the cell is made up of Teflon coated alloys. The container of the cell is divided into three compartments with the help of two graphite plates. These graphite plates are porous and contain catalysts like nickel, platinum, or palladium. The inner compartment is filled with a concentrated solution of KOH whereas lateral compartments have an entrance for hydrogen and oxygen gases. The central or inner compartments has an outlet for water produced during the course of the reaction. The Graphite plate which is in contact with hydrogen gas behaves as an anode whereas the graphite plate which is in contact with oxygen behaves as a cathode as shown above.
Working of H2-O2 Fuel Cell:
During the working of the cell, hydrogen gas ionizes at the anode to form H+ions and electrons. The H+ions pass through the graphite wall and reacts with OH–ions of KOH to form four molecules of water. Electron lost by the hydrogen passes to the outer circuit and reaches the cathode where they react with oxygen and two water molecules to produce four times OH–ions. These four OH–ions neutralize four K+ions in the central compartment i.e net reaction result in the production of 2H₂O.
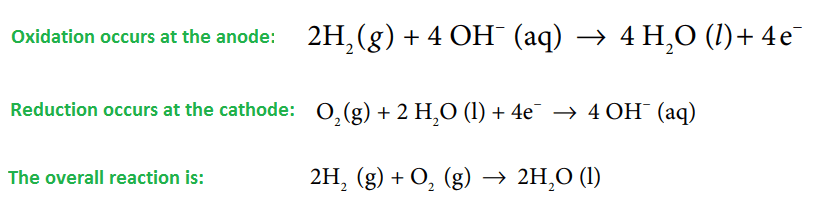
Advantages of Fuel Cell:
- This cell does not use or produce any kind of pollutant or containment.
- Efficiency of this cell is 65-75% compared to other cells having efficiency between 35-45%.
- Besides production of electricity the cell also produces H₂O.
- This cell is used in space ships and water produced in the cells is used for drinking purposes by astronauts after mixing with suitable salts.
Disadvantages of Fuel Cell:
- This cell is expensive for use in day-to-day life since the cell involves catalysts likes Nickel, Platinum, and Palladium which is very expensive.
- This cell uses hydrogen and oxygen the storage of which requires large cylinder because of low densities of the gases, which are not easily portable.
- Hydrogen is inflammable.









Comments (No)