Table of Contents
What is Biogeochemical Cycle?
The biogeochemical cycle may be defined as the more or less circular path which brings about the circulation of chemicals and elements, including all essential elements from the environment to the organisms and back to the environment.
Types of Biogeochemical Cycle:
Gaseous Cycles:
In which the reservoir is in the atmosphere. For example- carbon, hydrogen, oxygen, and nitrogen cycles.
Sedimentary Cycles:
In which the reservoir is in the lithosphere. For example- phosphorous, sulfur and calcium cycles.
Why are biogeochemical cycles essential to long-term life on Earth?
Biogeochemical Cycles are essential to long-term, sustainable life on Earth for several reasons-
- First, living things require many chemical elements, and these must be available at the right time, in the right amounts, and in the right concentrations relative to each other. This is the essence of, and the importance of, biogeochemical cycles.
- Second, sustained life on Earth is a function of ecosystems, and a primary ecosystem process is the cycling of chemicals necessary for life. On a larger scale, chemicals, nutrients, and trace elements necessary for life are made available from Earth through various parts of biogeochemical cycles. For example, soil and rock release nutrients to plants through weathering and biochemical processes; water infiltrates rock and soil to emerge as springs and streams necessary for life. In the ocean, single-cell algae release a sulfide compound that oxidizes in the atmosphere, producing condensation nuclei that are necessary to form clouds that transport water and sulfur to the land.
- Third, chemical reactions in biogeochemical cycles determine whether chemical elements and necessary compounds’ are available to living things. Photosynthesis involves the availability of carbon dioxide in the carbon cycle. Green plants use carbon dioxide with sunlight and water to produce sugar. A by-product is an oxygen, which is why we have free oxygen in the atmosphere. Without the carbon and water cycle, none of this would happen and life as we know it wouldn’t be possible.
Important Biogeochemical cycles:
Carbon Cycle In Nature:
Nitrogen Cycle In Nature:
Oxygen Cycle In Nature:
Water Cycle In Nature [Hydrological Cycle]:
Phosphorus Cycle In Nature:
Phosphorus is an important and necessary constituent of protoplast as it is an essential constituent of nucleic acids, phospholipids, and many phosphorylated compounds. The phosphorus tends to circulate as the organic compounds on being broken down produce phosphates which are again available to plants.
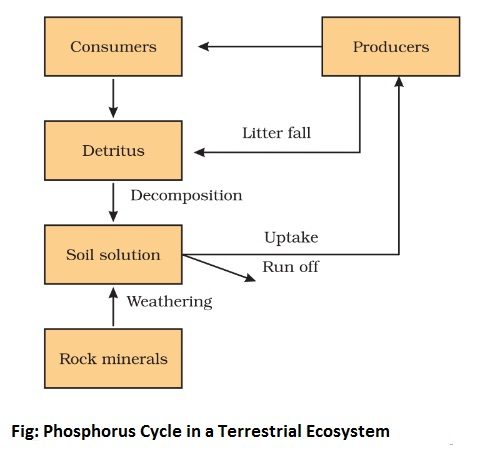
The crystalline rocks are the major source of phosphorus which occurs as phosphates. These rocks have been formed in past geological ages. These are gradually eroding, releasing phosphates to the ecosystems. Plants absorb the inorganic phosphates dissolved in water. These are transferred to animal consumers and decomposers as organic phosphates and subsequently, made available for recycling via mineralizing and decomposition. Much phosphate enters into the sea, where part of it is dissolved in the shallow sediments and part of it is lost to the deep sediments. There is no atmospheric phase in the phosphorus cycle. Phosphorus combined with metals like aluminum, iron, and calcium is not available to plants and is lost to the cycle. Phosphorus is incorporated in bones and teeth as calcium phosphate is resistant to decay. It may also remain outside the natural cycle for a long time.
Sulfur Cycle In Nature:
Sulfur is an essential constituent of amino acids cystine, cysteine, and methionine. It is also the structural component of several proteins. In nature, sulfur occurs in free form and also in the form of mineral salts. The main source of sulfur for the biological system is inorganic sulfate. Plants absorb sulfates from the soil dissolved in water and use it in protein synthesis. Animals get sulfur through the food. On the death of plants and animals, their bodies are decomposed under aerobic conditions by bacteria and fungi. Under anaerobic conditions, these are composed of microbes such as Aspergillus producing hydrogen sulfide which is oxidized by certain bacteria like Thiobaccillus into sulfates.
Burning of fossil fuels by industries and thermal power plants add sulfur dioxide (SO₂) in the atmosphere. With rainwater, it forms sulfurous acid (H2SO3) which on entering the living systems, interferes with the metabolic activities of the organisms.









Comments (No)