J.J. Thomson Method:
Principle- This method is based on the facts that-
- Stream of negatively charged particles (electrons) constitute current.
- The stream of negatively charged particles is deflected by both electric field as well as magnetic field.
Construction- It consists of highly evacuated glass tube G. At one end of the tube, filament F made of Tungsten is fitted. The filament F is heated with a low tension battery (L.T.), and hence it emits electrons. The emitted electrons are accelerated by high voltage V applied between anode A and cathode C.
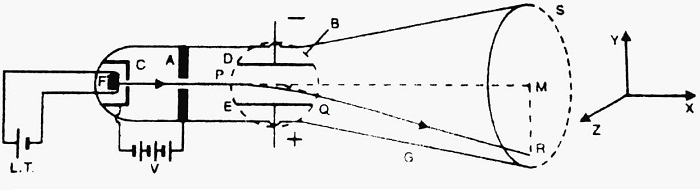
The accelerated electrons emerge through the hole in the anode in the form of a narrow beam. The electric field E can be applied perpendicular to the electron beam, with the help of metal plates D and E. The dotted circle represents the magnetic field. The magnetic field is applied perpendicular to both the electric field as well as to the electron beam. S is a screen coated with some fluorescent material ZnS. When the electron beam strikes the screen, a luminous spot is seen there.
Working and Theory-
Force acting on electron due to the electric field is given by-
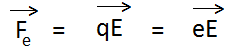
Force acting on electron due to the magnetic field is given by-
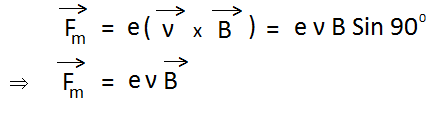
Suppose at the start, no electric field or magnetic field is applied. Hence the electron beam will go straight and a bright spot is seen at M. Apply both electric field and magnetic field. Now, the electron beam will be deflected and a bright spot is seen at R. Now adjust the values of the electric field and magnetic field, so that bright spot is again seen at M. At this stage,
| Fe = Fm ⇒ eE = eνB ⇒ ν = E/B ………………….(I) |
The K.E. gained by the electrons in going from cathode to the anode is given by-
| 1/2 mν2 = eV ⇒ ν2 = 2eV/m ⇒ ν = √2eV/m …………………..(II) |
| From (I) and (II), √2eV/m = E/B Square b/s ⇒ 2eV/m = E2/B2 ⇒ e/m = E2/2VB2 |
Knowing the values of E, V and B, the value of e/m can be calculated. It is found that the value of e/m comes to be 1.77 x 1011 c/kg. It should be noted that e/m is also called a specific charge of the electrons.








Comments (No)