Measurement of Electrolytic Conductance:
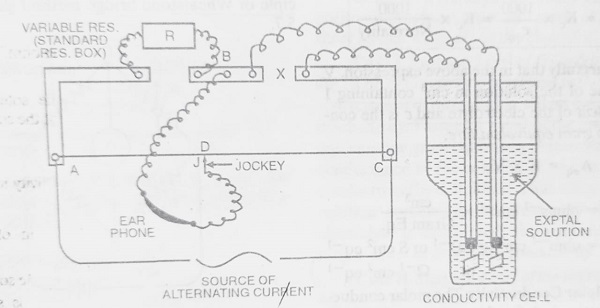
The conductance of the solution is reciprocal of Resistance and so if the resistance of the solution is known, its conductance can be calculated. The Resistance of the electrolytic solution is measured with the help of the Wheatstone Bridge method. It consists of four arms containing Resistances. The electrolytic solution whose conductance is to be determined is taken in a conductivity cell and is made one arm of the Wheatstone Bridge. This cell is made of Pyrex glass and is fitted with two Pt-electrodes at a fixed distance apart. The cell is placed in a thermostat to keep the temperature constant (because conductance changes with temperature). The connections are made as shown in the figure. The use of direct current causes electrolysis of the solution and the concentration of the solution changes leading to a change in the resistance of the solution. These effects are called “Polarization Effects”. To overcome this problem, an alternating current (A.C) of frequency range 1500-3000 cps (cycles per second) is used. However, when A.C is used, an ordinary Galvanometer fails to detect the “Null Point” (When there is no deflection in the Galvanometer, it is known as Null Point.) and it is replaced by an “earphone“. The Pt-electrodes are coated electrolytically with finely divided Pt-black to increase the sharpness of sound in the Null Point detector (earphone) i.e. to minimise polarization effects. The resistance box ‘R‘ constitute the second arm of the bridge. AC is a uniform wire stretched along a metre bridge. The bridge is connected to a suitable source of an alternating current like an oscillator.
A suitable value of resistance R is plugged out of the resistance box so that when the jockey ‘J‘ is moved along the stretched wire, the sound in the earphone is reduced to a minimum at a point (Say, D) somewhere in the middle of the wire AC. Then, if ‘X‘ is the resistance of the electrolytic solution, by the principle of Wheatstone Bridge,
| Resistance R / Resistance X = Resistance of Wire AD / Resistance of Wire CD = Length AD / Length CD Therefore, Resistance X = Resistance R x Length CD / Length AD |
| Therefore, Conductance, C = 1 / X = 1 / R x Length AD / Length CD |








Comments (No)