Millikan’s Oil Drop Experiment:
Principle- It is based upon measurement of:
- Terminal velocity of the oil drop under the action of gravity alone.
- Terminal velocity of the oil drop under the joint action of gravity and electric field opposing the gravity.
Construction- It consists of a double-walled chamber that is maintained at a constant temperature. The walls of the chamber contain some liquid. Light enters the chamber through window W1 to illuminate the field of view. X-rays enter the chamber through window W2 to ionize the drop.
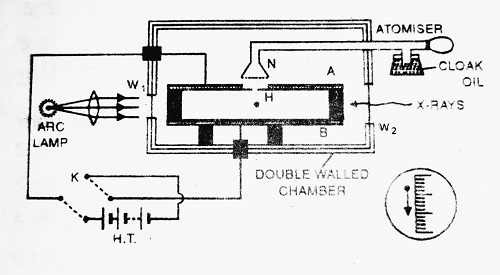
Two plates A and B each having diameter of about 22 cm and placed at a distance of 1.5 cm apart are held parallel with each other inside the chamber. The plate P has a fine hole H in the centre. A potential difference of about 10,000 volts is applied between plates A and B by the H.T. battery. Few drops of low vapour pressure oil (like cloak oil) are sprayed over hole H. Some of the oil drops may enter the space between two plates A and B. X-rays from window W2 charge these oil drops.
Working and Theory:
(I) When no electric field is applied- In this case, the oil drop moves under the action of gravity alone. Let ρ be the density of oil drop, σ be the density of air, η be the co-efficient of viscosity, r be the radius of oil drop and ν be the velocity of oil drop.
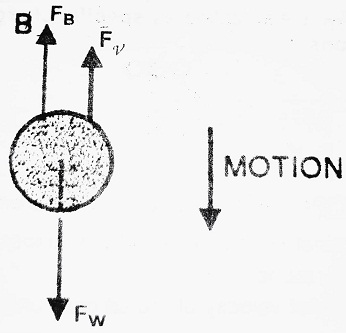
Various forces acting on the drop in this case are-
(1) Weight of the oil drop in a downward direction-
| (FW) = 4/3 πr3ρg ↓ |
(2) Buoyant force in the upward direction-
| (FB) = 4/3 πr3σg ↑ |
(3) Force due to viscosity in the upward direction-
| (Fν) = 6 πηrν ↑ |
At equilibrium, FW = FB + Fν
| ⇒ 4/3 πr3ρg = 4/3 πr3σg + 6 πηrν ⇒ 4/3 πr3ρg – 4/3 πr3σg = 6 πηrν ⇒ 4/3 πr3 (ρ – σ) g = 6 πηrν ………………..(1) ⇒ 2/3 r2 (ρ – σ) g = 3 ην ⇒ r2 = 9ην/2(ρ-σ)g |
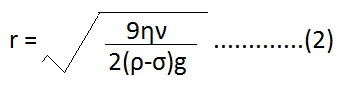
(II) When an electric field is applied- Now, apply potential between plates A and B in such a way that plate A will become positive.
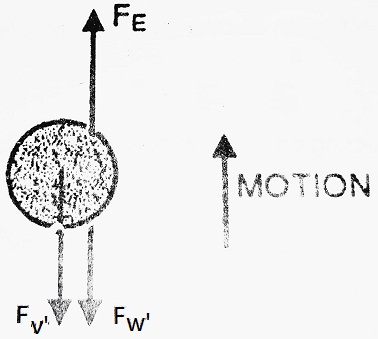
The oil drop, being negatively charged starts, moving upward instead of moving downward with velocity ‘ν’. In this case, oil drop moves under the combined action of gravity as well as an electric field. Various forces acting on the drop, in this case, will be-
(1) Force due to electric field in the upward direction
| (FE) = qE ↑ |
(2) Net weight of the drop in the downward direction
| (F’W) = 4/3 πr3 (ρ – σ)g ↓ |
(3) Force due to viscosity in the downward direction
| (F’ν) = 6 πηrν’ ↓ |
At equilibrium, FE = F’W + F’ν
| ⇒ qE = 4/3 πr3 (ρ – σ)g + 6 πηrν’ Put the value of 4/3 πr3 (ρ – σ)g from equation (1)- ⇒ qE = 6 πηrν + 6 πηrν’ ⇒ qE = 6 πηr (ν + ν’) ⇒ q = 6 πηr (ν + ν’)/E But E = V/d where ‘d’ is the distance between plates A and B. ⇒ q = 6 πη (ν + ν’)dr/E |
Put the value of ‘r’ from (2)-
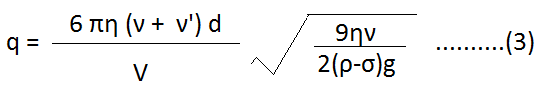
When we put the various values in equation (3), we can determine the charge of an electron. The charge of an electron from this experiment is found to be 1.602 x 10-19 C.
Precautions-
- A non-volatile oil (usually cloak oil) should be used.
- The air inbetween the plates should be homogeneous.
- The velocity of the droplet is to be recorded when it moves with constant speed.
Importance of Charge Determination:
(1) Millakan found that electronic charge was the smallest possible charge.
(2) Millikan found that charge on anybody was always an integral multiple of charge on the electron i.e. q = ne. Hence he established that the charge is quantized.
(3) It helps us to find the mass of the hydrogen atom. The mass of the hydrogen atom was found to be 1836 times the mass of an electron.
(4) When we combine the results of Millikan’s experiment and Thomson’s experiment, the mass of the electron can be calculated.
According to Millikan’s experiment-
| e = 1.602 x 10-19 C …………….(4) |
According to Thomson’s experiment-
| e/m = 1.758 x 1011 C/kg ………………….(5) |
Divide (4) by (5), we have-
| e/e/m = m = 1.602 x 10-19/1.758 x 1011 = 9.1 x 10-31 kg Hence, mass of electron, m = 9.1 x 10-31 kg |









Comments (No)