Table of Contents
What is Molecular Orbital?
When two atomic orbitals overlap, they lose their identity and form new orbitals known as Molecular orbitals which is defined as “the region in space defined by its size and shape associated with two or more atoms in a molecule and having a capacity of two electrons. Thus, a molecular orbital is polycentric. i.e., the electron is moving in the field of more than one nucleus.
Important Conditions Required by Atomic Orbitals To Form Molecular Orbitals:
Atomic orbitals can combine to form molecular orbitals only if the following conditions are satisfied-
- Energy of the Combining Atomic Orbitals- The energies of the combining atomic orbitals should not differ much from one another. Thus, 1s and 2s or 2s and 2p orbitals of two atoms of the same element will not combine to form molecular orbitals in homonuclear diatomic molecules of type A2. In heteronuclear diatomic molecules of the type AB, such combinations may be possible. Example in HF.
- Extent of Overlapping- The atomic orbitals can combine only if they can overlap to a considerable extent. This overlap brings the electron cloud in between the two nuclei and minimizes repulsion between them. Thus, greater is the overlap of atomic orbitals, greater is the build-up of charge between the nuclei causing greater attraction and thus will form a stronger bond.
- Symmetry about the Molecular Axis- The combining atomic orbitals should have the same symmetry about the molecular axis (i.e. the line joining the two nuclei). Example- 2s and 2px orbitals can overlap along X-axis but 2s and 2pz cannot overlap. However, they can overlap along Z-axis because both have the same symmetry about Z-axis. According to the modern concept, Z-axis is taken as an internuclear axis.
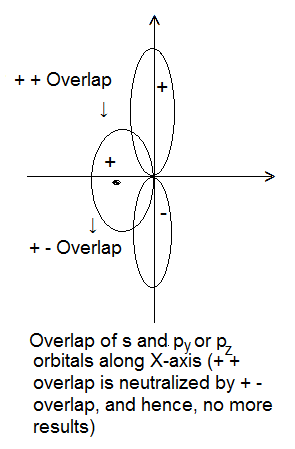
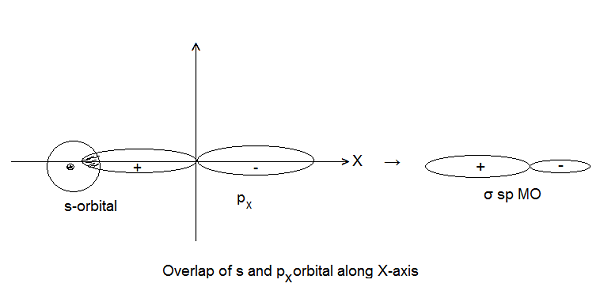
Formation of Molecular Orbitals (LCAO Method):
Due to the complex nature of the Schrodinger wave equation, it is not easy to solve it for molecules. For convenience, a new approximate method known as Linear Combination of Atomic Orbitals (LCAO) is used to obtain wave functions for molecular orbitals. Consider two similar atoms 1 and 2 with atomic orbitals described by the wave functions ψ1 and ψ2. When these atoms form a bond, the electrons originally present in atomic orbitals now occupy molecular orbitals which is formed by a linear combination of atomic orbitals ψ1 and ψ2 in two ways-
- By Addition of wave functions- When the two waves are in phase so that they add up and the amplitude of the new wave is-
| ψb = ψ1 + ψ2 ……………..(I) |
- By Subtraction of Wave Functions- When two waves are out of phase, they are subtracted from each other so that the amplitude of the new wave is-
| ψa = ψ1 – ψ2 ……………..(II) |
Since the probability of finding the electron in a particular region of space around the nucleus is given by the square of the wave function.
| Thus, ψb2 = (ψ1 + ψ2)2 = ψ12 + ψ22 + 2ψ1ψ2 ……………..(III) ψa2 = (ψ1 – ψ2)2 = ψ12 + ψ22 – 2ψ1ψ2 ……………..(IV) |
The term 2ψ1ψ2 arises due to overlapping and fusion of atomic orbitals.
Thus, the probability of finding the electron is more in molecular orbital formed according to the equation I (∵ ψb2 > ψ12 + ψ22) and the new orbital is known as Bonding Molecular Orbital (BMO). The probability of finding an electron in Molecular orbital formed by LCAO according to equation II is less than that in either of the atomic orbitals ψ1 and ψ2 and the new orbital formed is known as Antibonding Molecular Orbital (ABMO).
It is clear from the figure that Bonding Molecular Orbital (ψb) has lower energy than either of the atomic orbitals (ψ1 and ψ2) and thus causes the formation of a stable bond between atoms whereas Antibonding Molecular Orbital (ψa) has higher energy than either of the atomic orbitals and, hence, can not lead to the formation of a bond between the atoms.
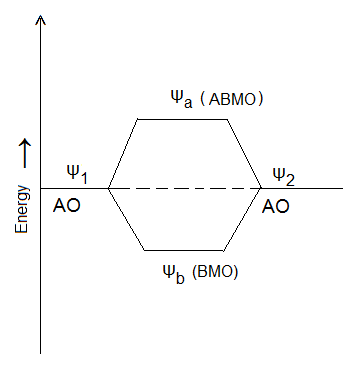









Comments (No)