Table of Contents
Organometallic Compounds:
Organometallic chemistry constitutes one of the most important areas of chemistry that unites the field of inorganic and organic chemistry together. As the name implies, organometallic compounds contain both a metal component as well as an organic component. Organometallic chemistry is that branch of chemistry that deals with compounds containing at least one direct metal-carbon bond. In other words, these are defined as those compounds which contain metal-carbon bond. But nowadays a broader definition for organometallics is used. According to which, these are defined as those compounds in which carbon is bonded to an element less electronegative than the carbon itself.
On the basis of these definitions, compounds like metal alkoxide (NaOC2H5), Sodium acetate (CH3COONa) which contain both metal as well as carbon but this bond is not direct. Hence these compounds are not organometallics.
Metal/metalloid – Carbon bond can be simple covalent, π-bond or predominately ionic. A few examples of organometallic compounds are as follows-
- (C2H5)4 Pb, covalent bond, (Tetraethyl Lead).
- Na+ C2H5, Ionic bond, (Ethyl Sodium).
Classification of Organometallic Compounds:
(1) σ-Bonded Organometallic Compounds- Most of the organometallic compounds contain metal-carbon sigma bond. Some of the examples of σ-Bonded Organometallic Compounds are as follows-
- CH3MgI (Methyl Magnesium Iodide).
- C6H5MgBr (Phenyl Magnesium Bromide).
Another interesting compound is trimethyl aluminium (CH3)3 Al. This compound occurs as a dimer with formula (CH3)6Al2 having the following structure.
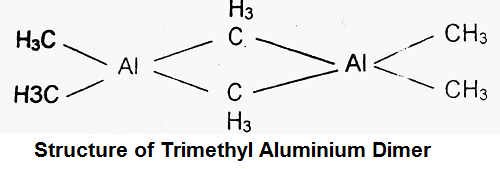
(2) π-Bonded Organometallic Compounds- These are usually formed by transition elements. An interesting compound of this type is bis(cyclopentadienyl) iron i.e. Fe(η5 – C5H5)2. Here η is called eta and it indicates that Fe is attached to all the five carbon atoms of the C5H5 species. This is called a Sandwich compound as iron is in the centre or in between the two organic rings.
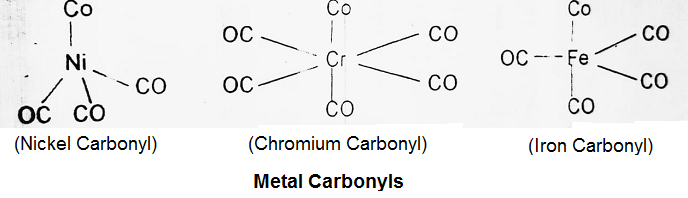
Metal Carbonyls:
The most studied class of compounds is metal carbonyl which are compounds of transition elements with carbon monoxide. Metal carbonyl contains both σ and π bonds. Some of the important metal carbonyls are nickel carbonyl, iron carbonyl, chromium carbonyl etc.
Applications of Organometallic:
(1) Organometallics have been used as a catalyst. They can be used as homogenous catalysts as well as heterogeneous catalysts.
Examples are-
- (Ph3P) RhCl Wilkinson Catalyst.
- Zigler Natta Type Catalyst.
(2) They can be used in the synthesis of organic compounds. Example- Grignard’s Reagent.
(3) Organometallic compounds have been used in the treatment of syphilis. Silicones have been used in cosmetics.








Comments (No)