Table of Contents
Oxidation and Reduction:
What is Oxidation?
When an element reacts with oxygen, an oxide is formed as a product.
Element + Oxygen ————–> Oxide
Such a reaction is called an oxidation reaction and the process in which the element is oxidized to form its oxide is known as Oxidation. At present, the term oxidation is taken in a broader sense in which it includes the addition of not only oxygen but of all elements or radicals which like oxygen are electronegative. It also includes the removal of hydrogen and all elements or radicals which like hydrogen are electropositive. Thus, oxidation may be defined as a chemical process which involves-
- addition of oxygen or any other electronegative element or radical.
- removal of hydrogen or any other electropositive element or radical.
Examples-
(i) Addition of oxygen.
S (sulfur) + O2 (oxygen) ——–> SO2 (Sulfur Dioxide)
2Zn (Zinc) + O2 (oxygen) ——–> 2ZnO (Zinc Oxide)
(ii) Addition of any other electronegative element or radical.
2FeCl2 (Ferrous Chloride) + Cl2 (Chlorine) ————> 2FeCl3 (Ferric Chloride)
2Al (Aluminium) + 3Cl2 (Chlorine) ———> 2AlCl3 (Aluminium Chloride)
(iii) Removal of Hydrogen.
H2S (Hydrogen Sulfide) + Cl2 (Chlorine) ————> S (Sulfur) + 2HCl (Hydrochloric Acid)
4NH3 (Ammonia) + 3O2 (oxygen) ——–> 2N2 (Nitrogen) + 6H2O (Water)
(iv) Removal of any other electropositive element or radical.
2KBr (Potassium bromide) + Cl2 (Chlorine) ————> Br2 (Bromine) + 2KCl (Potassium Chloride)
2KI (Potassium Iodide) + O3 (Ozone) + H2O (Water) ———> I2 (Iodine) + 2KOH (Potassium hydroxide) + O2 (Oxygen)
Oxidising Agent: A substance that causes oxidation is known as an oxidising agent. Thus, an oxidising agent supplies oxygen or any electronegative element or radical. The important oxidising agents are- oxygen, chlorine, bromine, ozone, hydrogen peroxide, nitric acid etc. An oxidising agent is also referred to as an Oxidant.
What is Reduction?
When the oxide of an element reacts with hydrogen, it is reduced to the corresponding element.
Oxide of an element + Hydrogen ————> Element + Water
Such a reaction is called a reduction reaction and the process in which the oxide of an element is changed into the corresponding element is known as reduction. The reduction is the reverse of oxidation and may, therefore, be defined as a chemical process which involves-
- removal of Oxygen or any other electronegative element or radical.
- addition of Hydrogen or any other electropositive element or radical.
Examples-
(i) Removal of Oxygen.
CuO (Cupric Oxide) + H2 (Hydrogen) ——-> Cu (Copper) + H2O (Water)
Fe2O3 (Ferric Oxide) + 3H2 (Hydrogen) ———-> 2Fe (Iron) + 3H2O (Water)
(ii) Removal of any other electronegative element or radical.
2FeCl3 (Ferric Chloride) + H2S (Hydrogen Sulfide) ———> 2FeCl2 (Ferrous Chloride) + 2HCl (Hydrogen Chloride) + S (Sulfur)
2FeCl3 (Ferric Chloride) ————> 2FeCl2 (Ferrous Chloride) + Cl2 (Chlorine)
(iii) Addition of Hydrogen.
Cl2 (Chlorine) + H2 (Hydrogen) ————> 2HCl (Hydrogen Chloride)
Cl2 (Chlorine) + H2S (Hydrogen Sulfide) ———–> 2HCl (Hydrogen Chloride) + S (Sulfur)
(iv) Addition of any other electropositive element or radical.
Br2 (Bromine) + 2Na (Sodium) ———–> 2NaBr (Sodium Bromide)
HgCl2 (Mercuric Chloride) + Hg (Mercury) ———> Hg2Cl2 (Mercurous Chloride)
Reducing Agent: A substance that causes reduction is known as a reducing agent. Thus, a reducing agent provides hydrogen or any electropositive element or radical. The important reducing agents are Hydrogen, Carbon, Stannous Chloride, Hydrogen Sulfide etc. A reducing agent is also referred to as a reductant.
Oxidation and Reduction go Simultaneously:
Oxidation and reduction always go side by side. In a reaction, one substance gets oxidised at the expense of another which is reduced and vice versa. For example-
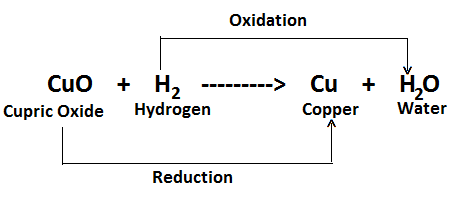
In this reaction, Hydrogen is oxidised to water while cupric oxide is simultaneously reduced to copper.
Electronic Concept for Oxidation and Reduction:
According to the electronic concept based on the knowledge of atomic structure, oxidation involves loss of electrons (de-electronation) and reduction involves a gain of electrons (electronation). Thus, these two terms may be defines as under-
Oxidation: It is a process in which an atom, a group of atoms or an ion loses one or more electrons. For example-
Na ——-> Na+ + e– ; Mg ———> Mg2+ + 2e–
Fe2+ ———> Fe3+ + e– ; 2Br– ———> Br2 + 2e–
Reduction: It is a process in which an atom, a group of atoms or an ion gains one or more electrons. For example-
S + 2e– ———–> S2- ; Cl2 + 2e– ———–> 2Cl–
Fe2+ + 2e– ———> Fe ; Fe3+ + e– ———-> Fe2+
An atom, a molecule, or an ion that gains electrons is called an oxidising agent and one which loses electrons is called a reducing agent.
Since oxidation and reduction occur simultaneously, oxidation and reduction reaction (redox reaction) involves loss of electrons as well as gain of electrons. For example-
Mg —————> Mg2+ + 2e–
F2 + 2e– ——————-> 2F–
Mg + F2 —————–> Mg2+ + 2F– or Mg2+(F–)2 or MgF2
Limitations of Electronic Concept:
The electronic concept of oxidation and reduction is applicable only to the reaction involving ions i.e. when the reaction involves ionic compounds. Difficulties arise when it is extended to covalent compounds, which are formed by the sharing of electrons. In the case of covalent compounds, oxidation-reduction is explained on the basis of the oxidation number concept.









Comments (No)