Table of Contents
Photoelectrolysis of Water:
Hydrogen is a very good source of energy and it has an advantage over other fuels like petrol, cooking gas or coal because its combustion does not produce CO, CO2 or some other poisonous by-product and is, therefore free from pollution. Since water is an inexhaustible source of energy and efforts are being made to split up H2O into H2 and O2 using solar energy. One of the most important such methods is known as “Photoelectrolysis of Water“. It involves the electrolysis of water using Photosensitive semiconductor electrodes (Cathode and Anode) in a Photoelectrolysis Cell which make use of one electrode or both the electrodes capable of providing electrons for reduction (act as a cathode) or remove electrons for oxidation (act as an Anode) instead of Voltaic Cell. The two commonly used Photoelectrolysis Cells are-
Cells having both Semi-Conductor Photo Cathode and Semi-Conductor Photo Anode:
In this cell, an aqueous solution of NaOH or Na2SO3 is used as the electrolyte, the cathode is a P-type photosensitive semi-conductor like GaP (Gallium phosphide) and Anode is an N-type photosensitive semi-conductor like TiO2. In some photoelectrolysis cells, the cathode and anode are made of the same semiconductor material, one side of which is dropped with N-type impurity and the other with P-type. Example- Pellets of Fe2O3 dopped with Si4+ would become N-type semiconductor and dopped with Mg2+ will become P-type semiconductor. Fe2O3 dopped with Mg2+ and Si4+ ions and cemented back to back becomes such an electrode system. When electrodes in such cells are exposed to radiations, H2O is oxidized at the Anode evolving O2 gas and reduced at the cathode with the evolution of H2 gas.
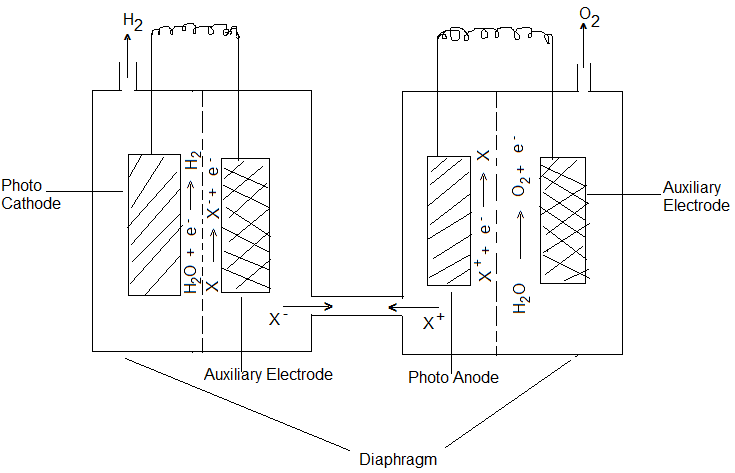
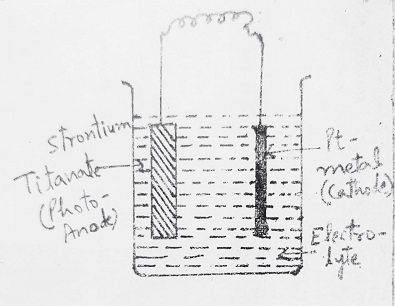
Cells having one Photosensitive N or P-type Semi-Conductor electrode and the other metal counter electrode:
In such cells, a suitable aqueous solution of an electrolyte (NaOH) is used, the Anode is a photosensitive semiconductor electrode like N-TiO2 and the cathode is Pt. In such a cell, it is necessary to apply external voltage in addition to normal solar energy exposure. But if SrTiO3 (Strontium Titanate) is used as a photoanode (in place of TiO2) no external voltage is required and electrolysis occurs only by light energy.
Maximum efficiency of 20-25% has been achieved in Photoelectrolysis by using oxygen-deficient N-TiO2 or alloying with VO2 and using radiation of 335 nm.







Comments (No)