Table of Contents
Dry Cell and Mercury Cell:
Primary Cells:
This type of cells can be used only so long as the active materials are present in the cells. Once these are consumed, the chemical reaction stops and the cell cannot be recharged or used again. The two most common primary cells are the Dry Cell and Mercury Cell.
Dry Cell:
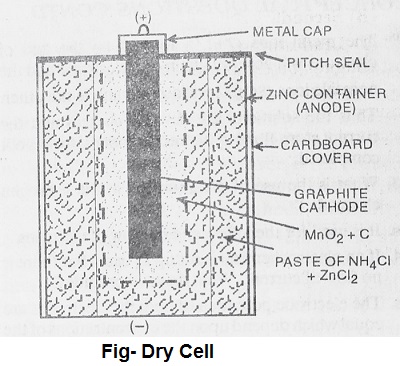
It is a compact form of Leclanche Cell in which anode consists of Zn container and Cathode is a graphite rod surrounded by powdered MnO2 and carbon. The space between the electrodes is filled with a moist paste of NH4Cl and ZnCl2. The electrode reactions are complex and can be written in the simplified form as follows-
At Cathode: MnO2 + NH4+ + e– ———-> MnO(OH) + NH3
{Oxidation state of Mn decreases from +4 to +3}
At Anode: Zn (s) ———-> Zn+2 + 2e–
The Zn+2 ions combines with NH3 liberated at cathode to form diammine Zn (II) cation.
Zn+2 + 2NH3 ———> [Zn(NH3)]+2
Dry cells do not have a long life as acidic NH4Cl corrodes the Zn container even if the cell is not in use. The cell potential of dry cells lies in the range 1.25-1.5 V.
The efficiency of a Dry Cell- The thermodynamic efficiency (i.e efficiency of energy conversion) in a reversible process is the ratio of energy available for useful work to the total energy change i.e.
Efficiency, η = Energy available for useful work / Total energy change in the system
η = ΔG / ΔH = -nFE / ΔH
The efficiency of energy conversions is not 100% because ΔG is less than ΔH. The efficiency of a dry cell has been found out to be 70%.
Mercury Cell:
It is a miniature cell commonly used in hearing aids, watches, cameras etc. In this cell, Zinc amalgam acts as an anode and the cathode is a paste of HgO and Carbon. The electrolyte is a moist paste of KOH and ZnO. The cell reactions are-
At Anode: Zn (from Zn-amalgam) + 2OH– ———-> ZnO (s) + H2O (l) + 2e–
At Cathode: HgO (s) + H2O (l) + 2e– ————> Hg (l) + 2OH–
Net Reaction: Zn + HgO (s) —————> ZnO (s) + Hg (l)
Since the overall cell reaction does not involve any ion in a solution whose concentration can change, the cell shows a constancy in Potential throughout its life and the cell potential ≃ 1.35 V.








Comments (No)