Table of Contents
Rutherford Gold Foil Experiment:
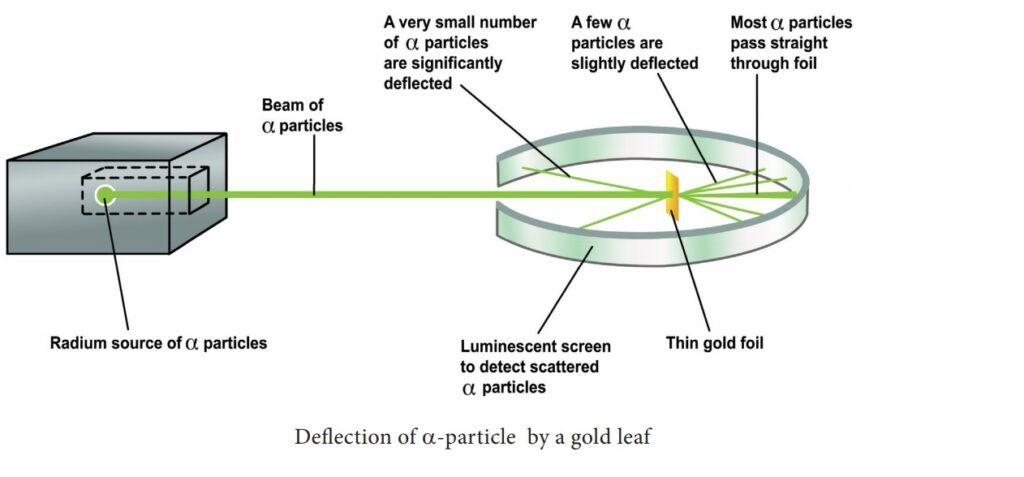
The evidence for the existence of the nucleus has been provided by Rutherford’s Experiment which he carried out in 1911. In his experiment, he bombarded a thin gold foil with a beam of alpha particles (particles carrying two units of positive charge and four units of mass) and observed their behavior with the help of a screen coated with zinc sulfide. Whenever an alpha particle struck the screen, a tiny flash of light appeared at the point of contact. He found that most of the alpha particles passed very nearly straight through the foil and struck the screen. Some of them were scattered at quite large angles from their path and very few retraced their path back. Rutherford interpreted these observations as follows:
- As most of the alpha particles passed very nearly straight through the foil, the atoms, far from being solid spheres, are extraordinarily empty.
- As some of the alpha particles were deflected from their path through large angles, there must be present in each atom a small central part in which its positive charge and mass are concentrated. This small positively charged and massive central part of the atom is called the nucleus.
- As a very small proportion of alpha particles retraced their path back, the nucleus must be a rigid one so that it can recoil the alpha particles.
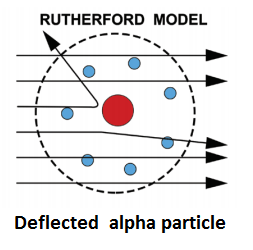
On the basis of these conclusions, Rutherford proposed that an atom consists of-
- A minute positively charged central part called the nucleus, in which almost the entire mass of the atom is concentrated.
- Electrons, sufficient in number to balance the positive charge on the nucleus. This part of the atom is named as the extra-nuclear part.
To account for the stable picture of the atom, Rutherford proposed that the electrons surrounding the nucleus are not stationary but revolving in closed orbits. The centrifugal force arising from the motion of electrons just balances the force of electrostatic attraction between them and the nucleus and thus the electrons remain in their path.
In 1913, Niels Bohr stated that the picture of the atom as suggested by Rutherford could not be stable. He said that the electrons revolving about the nucleus will be gradually losing energy and, thus, the orbits will become smaller and smaller till they fall into the nucleus. To solve this problem. he studied the motion of electrons in the light of quantum theory according to which energy is gained or lost not continuously but in bundles or quanta and no energy less than a quantum can be gained or lost. A quantum of energy is given by the relation E = hν, where ‘ν‘ is the frequency of radiation absorbed or emitted and ‘h’ is Planck’s constant. The numerical value of this constant is 6.62 × 10-34 Js. He postulated that the electrons in an atom move about the nucleus only in certain fixed or stationary orbits (also called shells) and the revolving electrons in the orbits are associated with a definite amount of energy. He, thus, also named the orbits as energy levels. According to Bohr, normally an electron remains revolving in a stationary orbit without losing or gaining energy and if it loses or gains energy as the conditions demand then it can do so only by emission or absorption of a quantum of energy and never less than that. Further, an electron can jump from a higher energy level to a lower energy level by emitting quantized energy but in case it is in the lowest energy level, the level nearest to the nucleus, it is not in a position to emit energy. Thus, the atom does not collapse.
With the discovery of neutrons, the present picture of the nucleus is that it contains protons and neutrons. Since the atom as a whole is electrically neutral, the number of protons in the nucleus is exactly the same as the number of electrons that revolve round the nucleus.
What is Atomic Number?
The atomic number of an element is given by the number of unit positive charges on the nucleus of its atom, which is equal to the number of protons present in the nucleus. For example, nitrogen has 7 protons in the nucleus of its atom and hence its atomic number is 7. In a neutral atom, the number of protons in the nucleus ie equal to the number of electrons in the extra-nuclear part. Thus-
Atomic Number (Z) = Number of Protons = Number of Electrons
What is Mass Number?
The mass number of an atom is equal to the number of protons plus the number of neutrons in its nucleus. Thus,
Mass Number (A) = Number of Protons + Number of Neutrons
The mass number is always a whole number. Knowing the atomic number and mass number, we can calculate the number of neutrons present in the atom of an element. For example- sodium with atomic number 11 and mass number 23 has 23 – 11 = 12 neutrons in its atom.









Comments (No)