What is Surface Tension?
Surface Tension may be defined as the property of the liquid by virtue of which the free surface of the liquid at rest tends to have minimum area and as such, it behaves as if covered with a stretched membrane.
In other words it may be defined as the tensile force acting on the surface of a liquid in contact with a gas or on the surface between two immiscible liquids such that the contact surface behaves like a membrane under tension. The magnitude of this force per unit length of the free surface will have the the same value as the surface energy per unit area. It is denotd by Greek letter σ. In MKS units, it is expressed as Kgf/m while in SI units as N/m.
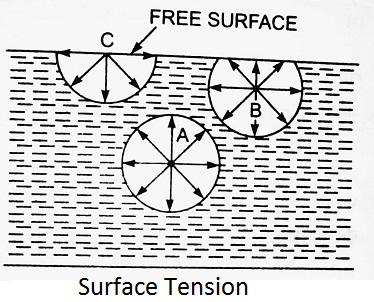
The phenomenon of surface tension is explained in figure. Consider three molecules A, B, C of a liquid in a mass of liquid. The molecule A is attracted in all directions equally by the surrounding molecules of the liquid. Thus the resultant force acting on the molecule A is zero. But the molecule B, which is situated near the free surface, is acted upon by upward and downward forces which are unbalanced. Thus a net resultant force on molecule B is acting in the downward direction. The molecule C situated on the free surface of liquid, does experience a resultant downward force. All the molecules on the free surface experience a downward force. Thus the free surface of the liquid acts like a very thin film under tension of the surface of the liquid act as though it is an elastic membrane under tension.
Factors Affecting Surface Tension:
(1) Temperature- Surface tension decreases with the rise in temperature and vanishes at the critical temperature for some liquids. Many formulae have been proposed to give variation of surface tension with temperature but none has been found completely satisfactory. The decrease in surface tension with temperature is due to increase in the average separation between the molecules.
(2) Contamination- Contamination due to oil, grease, dust etc., reduces the surface tension of water considerably.
(3) Concentration- In case of solutions, the surface tension depends on the degree of concentration of the solution. Whether the surface tension will increase or decrease depends on the nature of the solute. If the solute is very soluble, then the surface tension increases, as for example dissolving salt in water. If the solute is less soluble, then the surface tension decreases, for example the addition of soap or phenol decreases surface tension of water.
(4) Electrification- When a liquid is electrified, the effect is as if its surface tension has decreased. This is so because due to electrification the liquid surface experiences an outward normal pressure tending to increase the surface area. It is on account of this that a soap bubble tends to expand on electrification.
(5) Surroundings- The surface tension of a liquid depends on the nature of the media in contact with its surface.









Comments (No)