Wave Nature of Electron (Prove by Davison and Germer Experiment):
The wave nature of electrons has been established experimentally by Davison and Germer in 1927.
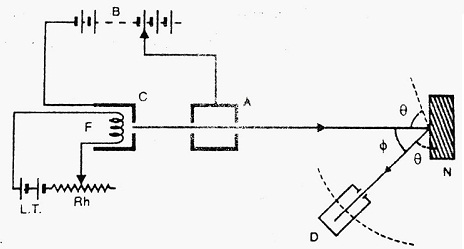
The apparatus consists of a Filament ‘F’ made up of Tungsten, which on heating with a low tension battery (L.T.) emits a large number of electrons. ‘A’ is an anode with a fine hole. The beam of electrons emitted from the cathode is allowed to pass through the hole in the anode. ‘N’ is a nickel crystal cut along a cubical diagonal. ‘D’ is an electron detector. It can be rotated on the circular scale and is connected to a sensitive galvanometer, which records current.
Working- A fine beam of electrons is made to fall on a nickel crystal. The incident electrons are then scattered in different directions by the atoms of nickel crystal. By rotating the electron detector on a circular scale, the intensity of the scattered beam is measured at different latitude angle Φ.
Polar graphs are then plotted between the intensity of scattered electrons and latitude angle Φ for different accelerating voltages from 44 volts to 68 volts. The graphs show that there is a sharp bump when the accelerating voltage is 54 volts and Φ = 50°.
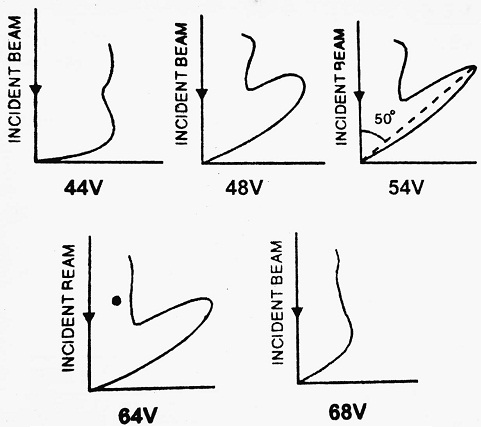
The appearance of a bump in a particular direction is due to the constructive interference of electrons scattered from nickel crystal. This establishes the wave nature of electrons.
| From simple geometry, and for Φ = 50°. θ + Φ + θ = 180° ⇒ 2θ + Φ = 180° ⇒ 2θ = (180° – Φ) ⇒ 2θ = (180° – 50°) = 130° ⇒ θ = 65° |
Also, for nickel crystal, the interatomic separation d = 0.91 Å.
According to Brag’s Law, and for 1st order diffraction maxima (n=1)
| 2d Sinθ = nλ ⇒ 2d Sinθ = 1 x λ ⇒ 2 x 0.91 x Sin 65° = λ ⇒ λ = 1.65 Å |
According to de Broglie hypothesis, the wavelength of wave associated with electron is given by-
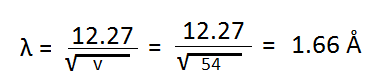
This shows that there is close agreement between an estimated value and experimental value. This proves the existence of de Broglie waves for the electrons in motion.







Comments (No)