Table of Contents
Bonding in Coordination Compounds:
Werner’s theory was the first successful attempt to describe the nature of bonding in coordination compounds. However, the simplest theory which explains the nature of bonding within the coordination sphere was proposed by Linus Pauling (1931) and is known as Valence Bond Theory. According to this theory-
- The central metal loses a requisite number of electrons (equal to its valency) to form the cation.
- The cation provides a number of empty orbitals (equal to its coordination number) for the formation of covalent (coordinate) bonds with the ligands.
- The cation orbitals i.e. s, p or d (depending on the total number of bonds to be formed with the ligands) hybridise to form a new set of equivalent bonding orbitals having definite directional properties known as hybridized orbitals.
- The non-bonding metal electrons present in the inner d-orbitals do not take part in hybridization (i.e. in chemical bonding).
- The empty hybrid orbitals of the cation overlap with those of the ligand orbitals that can donate an electron pair for bonding forming ligand —-> metal coordinate bonds. (The number of bonds formed is equal to the number of empty orbitals provided by the central metal ion i.e. cation).
- The d-orbitals involved in hybridization may be inner (n-1)d or outer ‘nd‘ orbitals. The complexes formed by using inner d-electrons are known as Inner orbital or Low spin complexes while those formed by using outer d-electrons are known as Outer Orbital or High Spin Complexes (because they contain a higher number of unpaired electrons).
- The complexes containing unpaired electrons are paramagnetic while those which do not contain unpaired electrons are diamagnetic in nature.
- The electrons can be forced to pair up against the Hund’s rule of maximum multiplicity under the influence of a strong ligand.
Examples to illustrate the above features of Valence Bond Theory:
Tetrahedral Complexes:
The formation of these complexes involves sp3 hybridization of the central metal ion (such as Cu+, Co2+, Fe2+, Ni, Zn2+) whose coordination number is 4. For example– in the complex Tetracarbonyl Nickel (0) i.e. [Ni (CO)4], the electronic configuration of the central metal Ni is 3d8, 4s2. In the presence of CO (a strong ligand), rearrangement takes place and the electrons are paired up against Hund’s rule. The two 4s electrons go to the 3d orbitals in order to make available the 4s orbitals for electrons donated by CO ligand. Thus, one 4s and three 4p orbitals hybridize giving four sp3 hybridized orbitals. Each CO molecule donates a pair of electrons to form a tetrahedral complex. It contains no unpaired electrons and is thus Diamagnetic (which supports the pairing of 4s electrons with 3d electrons). The hybridization and formation of the complex is shown below-
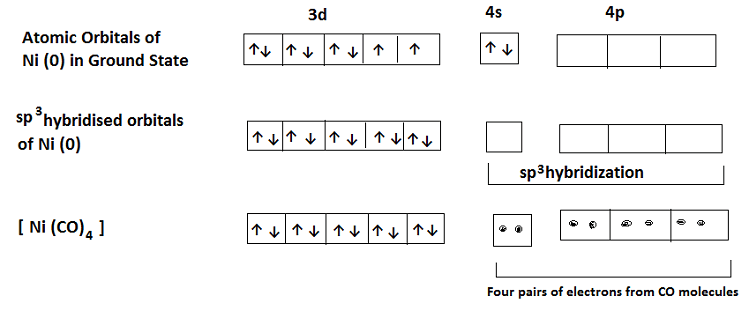
Square Planar Complexes:
These complexes involve dsp2 hybridization of the central metal ion (such as Cu2+, Ni2+, Pd2+, Pt2+, Au3+) whose coordination number is 4. In this type, one d, one s and two p orbitals hybridize to form four equivalent hybrid orbitals which are planar and mutually at right angles pointing towards the corners of a square. These complexes are inner orbital complexes. For example- in the complex Tetracyanonickelate (II) i.e. [Ni (CN)4]2-, Ni is in +2 oxidation state and has the configuration 3d8. The CN– ligand is strong and forces the pairing of 3d electrons making it available for hybridization with 4s and two 4p orbitals giving four dsp2 hybridized orbitals which accommodate the four pair of electrons from the ligands (CN–). It contains no unpaired electron and is Diamagnetic. The hybridisation scheme is shown below-
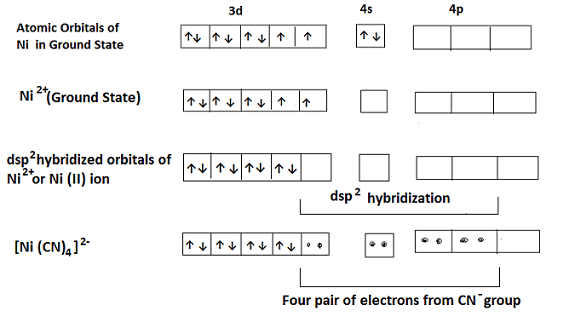
Octahedral Complexes:
These complexes involve sp3d2 or d2sp3 hybridization of the central metal ion (such as Ni2+, Fe2+, Fe3+, Co3+, Cr3+, Pt4+, Pd4+) whose coordination number is 6. It involves the combination of one s, three p and two d orbitals to form six hybridized orbitals having octahedral geometry. These complexes may be inner orbital (d2sp3) or outer orbital (sp3d2). Example- (i) In the complex hexacyanoferrate (II) i.e. [Fe (CN)6]4-, Fe is in +2 oxidation state and has the configuration of 3d6. The CN– ligand is strong and forces the pairing of 3d electrons making two 3d orbitals available for hybridization with 4s and three 4p orbitals giving six d2sp3 hybridized orbitals which accommodate the six electron pairs from the ligands (CN–). It contains no unpaired electron and is diamagnetic. It is an inner orbital complex as it involves d-orbital of the inner shell i.e. 3d. The hybridization scheme is shown below-
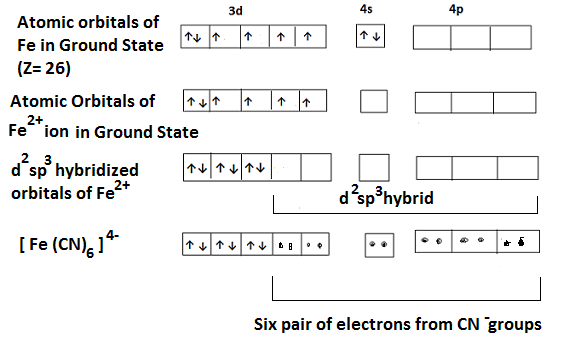
(ii) In the complex hexafluorocobaltate (III) ion i.e. [CoF6]3-, Co is in +3 oxidation state and has the configuration 3d6. Since F– ion is a weak ligand and two 4d orbitals hybridize giving six sp3d2 hybrid orbitals. The six F– ions donate a pair of electrons to each of these vacant orbitals giving octahedral shape to the complex. It is an outer orbital complex. The presence of unpaired electrons in 3d orbitals indicate that is Paramagnetic in nature. The hybridization scheme is shown below-
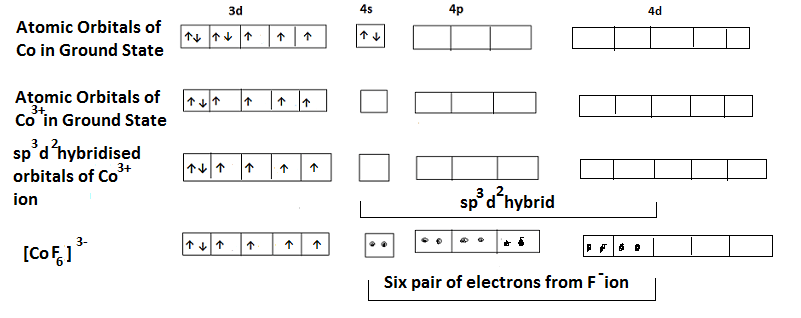
Limitations of Valence Bond Theory:
- It could not explain detailed magnetic properties of the complexes.
- It could not explain the formation of low spin and high spin complexes properly.
- It does not give any exact explanation of stability of coordination compounds.
- It does not distinguish between weak field and strong field ligands.
- It does not give any explanation about the colour of the complexes.









Comments (No)