Table of Contents
Werner Theory of Coordination Compounds:
Alfred Werner in 1893 proposed the first most successful theory to explain the formation and properties of coordination or complex compounds in terms of their structures known as Werner’s Coordination Theory and he was awarded Nobel Prize in chemistry for his theory.
The salient features of this theory are as follows-
(i) Metals have two types of valencies (or linkages) known as- (a) Primary or Principal or Ionisable Valency and (b) Secondary or Auxiliary or Non-ionisable Valency.
(ii) In modern terminology, the primary valencies usually indicate the oxidation number of the metal ion whereas secondary valencies represent the coordination number of the metal.
(iii) The primary valency of the central metal ion is always satisfied by anions and its attachment to the metal is shown by dotted lines. The secondary valencies may be satisfied either by anions or by neutral molecules and their attachment to the metal is shown by thick lines. For example- NH3 is added to cobalt chloride, a complex CoCl3.6NH3 is obtained, in which primary valencies of Co are satisfied by three chloride ions whereas the secondary valencies are satisfied by six NH3 molecules.
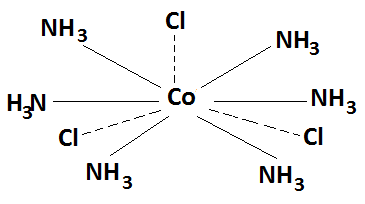
(iv) The secondary valencies from the coordination sphere of the complex and are always shown is square brackets and they do not ionise in solution to give constituent ions of the complex ion.
(v) Every metal has a fixed number of secondary valencies and it must satisfy all its primary and secondary valencies. In order to fulfil this requirement, an anion may perform dual behaviour of satisfying both the Primary and Secondary valencies and their attachment is shown both by dotted as well as thick lines. Such valencies which perform a dual role are not ionised. For example- the complex like CoCl3.5NH3, CoCl3.4NH3, CoCl3.3NH3 are represented with complex species shown within the square brackets (coordination sphere) and ionisation ligands (primary valency) kept outside the sphere i.e.
| S.No. | Werner Formula | No. of ions | Modern Formula |
|---|---|---|---|
| (i) | CoCl3.5NH3 | 3 | [Co(NH3)5Cl]Cl2 |
| (ii) | CoCl3.4NH3 | 2 | [Co(NH3)4Cl2]Cl |
| (iii) | CoCl3.3NH3 | 0 | [Co(NH3)3Cl3] |
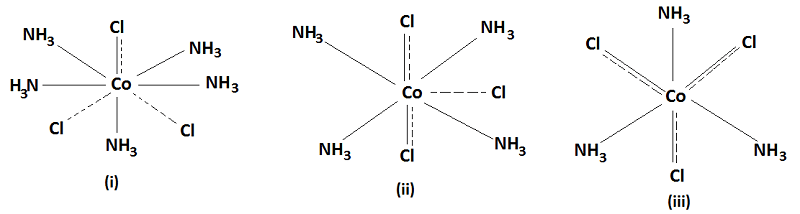
(vi) The primary valencies are non-directional whereas the ligands satisfying the secondary valencies are always directed towards fixed positions in space giving rise to stereo-isomerism in complex compounds. These coordinated groups arrange themselves around the metal ion in a planar, tetrahedral or octahedral manner depending upon the coordination number of the metal ion. For example- when the coordination number is 4, the arrangement is either square planar or tetrahedral while it is octahedral when the coordination number is 6.
Disadvantages of Werner’s Coordination Theory:
- There is no theoretical explanation for bonding because electrons and noble gases which form the bases for bonding are not known at that time.
- This theory does not explain the various facts like colour, magnetic behaviour etc.
- This theory does not explain why certain elements form coordination compounds and not others.
- It does not explain why the coordination sphere has a definite geometry.
- It does not explain the optical properties of compounds.
Types of Hybridizations and Geometries in Coordination Complexes:
| Hybridization | Geometry |
|---|---|
| sp3 | Tetrahedral |
| dsp2 | Square Planar |
| sp3d2 or d2sp3 | Octahedral |
| dsp3 | Trigonal Bipyramidal |
| sp3d | Square Pyramidal |






Comments (No)