Table of Contents
Electrochemical Theory of Rusting:
What is Corrosion?
It has been found that certain metals are slowly eaten up on long exposure to the atmosphere. Example- rusting of iron, tarnishing of silver, deposition of the green coating on copper and bronze etc. is known as Corrosion and is defined as the process of slow conversion of metals into their unwanted compounds (usually oxides) by reaction with moisture and other gases present in the atmosphere. More active metals are more prone to Corrosion and impurities also enhances the rate of corrosion. The presence of gases like SO2 and CO2 in air and electrolytes like saline water on the surface of metal also increases the rate of corrosion.
Mechanism of Corrosion:
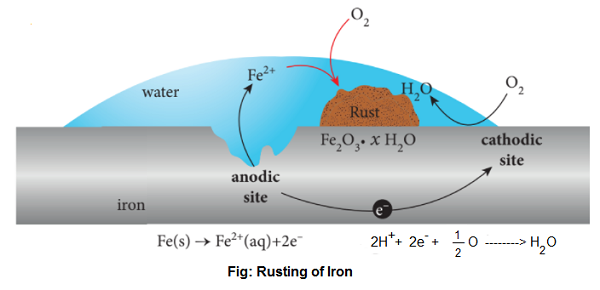
In Corrosion, a metal is oxidized by the loss of electrons to oxygen and forms metal oxide. Example- corrosion of iron i.e. Rusting is caused by moisture, CO2 and O2 of air. Rust is a non-sticking brown coloured material that can be removed by scratching and chemically, it is hydrated iron (III) oxide (Fe2O3.xH2O). The most widely accepted theory of rusting is known as the “Electrochemical Theory of Rusting“. According to this theory, the impure Fe surface behaves like a small electrochemical cell in the presence of H2O, containing dissolved O2 or CO2 known as miniature corrosion cell in which pure iron acts as Anode and impure surfaces act as cathode. Moisture having dissolved O2 or CO2 constitutes the electrolyte.
| At anode, Fe ——> Fe+2 + 2e–, E°ox = +0.44 V |
Thus, Fe atoms pass into solution as Fe+2 ions and the electrons produced move into cathodic area.
At the cathode, the electrons are taken by H+ ions which are produced either from H2O or H2CO3 (formed by dissolution of CO2 in moisture).
| CO2 + H2O ————-> H2CO3 ————-> H+ + HCO3– 2H+ (aq) + 2e– ————-> 2H |
The H-atoms reduce dissolved O2 to water.
| 2H + 1/2O2 ————-> H2O |
The net cathodic reaction is-
| 2H+ + 1/2O2 + 2e– ————-> H2O, E°red = 1.23 V |
Thus, the net reaction of the corrosion cell is-
| Fe + 2H+ + 1/2O2 ———–> Fe+2 + H2O, E°cell = 1.67 V |
The Fe+2 ions so formed move through the water and come at the surface of an iron object where these are oxidized to the ferric state by atmospheric oxygen forming rust which is hydrated-iron (III) oxide.
| 2Fe+2 + 1/2O2 + 2H2O ————-> Fe2O3 + 4H+ Fe2O3 + xH2O —————-> Fe2O3 . xH2O (Rust) |
Prevention Of Corrosion:
Some of the important methods for protecting metals from corrosion are-
Barrier Protection:
In this method, a barrier film is introduced between iron and atmospheric air by any of the following methods-
- By painting the surface.
- By coating the surface with a thin film of oil or grease.
- By electroplating iron with some non-corrosive metal like Ni, Cr etc.
Sacrificial or Electrical Protection:
In this method, the surface of iron is covered with more electropositive metals like Zn, Al, Mg etc. which loses electrons in preference to iron and hence, prevents the rusting of iron. Zinc is generally used for protecting iron and the process is known as Galvanization. Galvanized iron sheets maintain their shine due to the formation of a thin protective layer of basic Zinc carbonate [ZnCO3 . Zn(OH)2] due to reaction between Zn, O2, CO2 and moisture in the air. Instead of coating the more reactive metals on iron, rusting can also be prevented by connecting iron to a nearby buried Zn or Mg with the help of a metallic wire. Here, Fe behaves as Cathode and more reactive metal (Mg or Zn) as Anode. The electrons lost by more reactive metal are gained by H+ ions or H2O molecules at the surface of the iron. To prevent corrosion over a long period of time, the buried reduction metal may be replaced from time to time.






Comments (No)