Phase Diagram of Helium:
Helium is the only known pure substance that can exist in two different isotropic liquid phases i.e., Helium (He) is the only substance with a liquid-liquid boundary in its phase diagram. He exists in two liquid forms, liquid He-I, and liquid He-II.
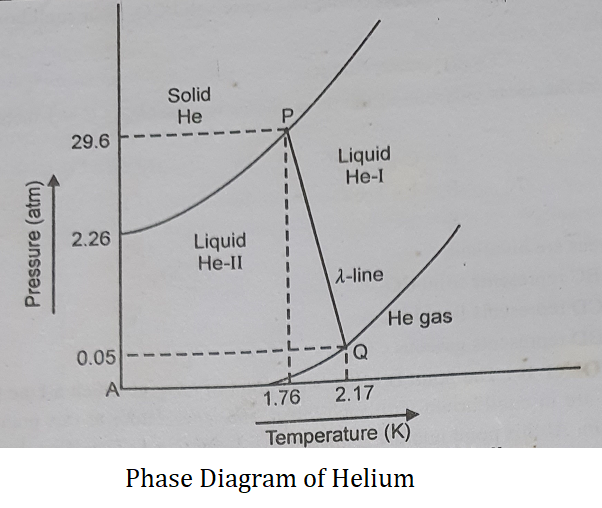
The following conclusions can be drawn from the phase diagram.
(1) Irrespective of how low the temperature is, the solid and gas phases of helium are never in equilibrium. This is because the helium atoms are so light that they vibrate with large amplitude motion even at very low temperatures. As a consequence, the solid simply shakes itself apart. That’s why, Helium remains as a liquid even at 0 K below 25 atm.
(2) There are two triple points P and Q.

(3) The transition between He-I and He-II in the presence of He vapor is known as lambda transition. The transition point is called lambda point (Q). The λ line marks the conditions under which the two liquid phases are in equilibrium.
(4) Liquid He-I behaves like a normal liquid. Liquid He-II displays unusual properties. It has an extremely low viscosity. When liquid He-II is put into an open container it spontaneously overflows and the solid simply shakes itself apart. That is why it is known as superfluid.
(5) By applying pressure we can hold He atoms together to obtain solid Helium.








Comments (No)