Relationship Between Molality and Mole Fraction:
| Molality = (1000 . x2) / (x1 . M1) |
Where x1 = Mole fraction of solvent.
x2 = Mole fraction of solute.
M1 = Molecular mass of solvent.
We know that,
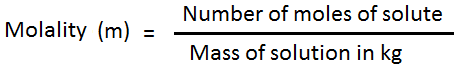
So, molality ‘m’ means ‘m’ moles dissolved in 1000 gm of solvent; (Which has a number of moles = 1000/M1; where M1 = molecular mass of solvent).
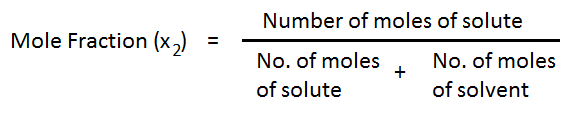
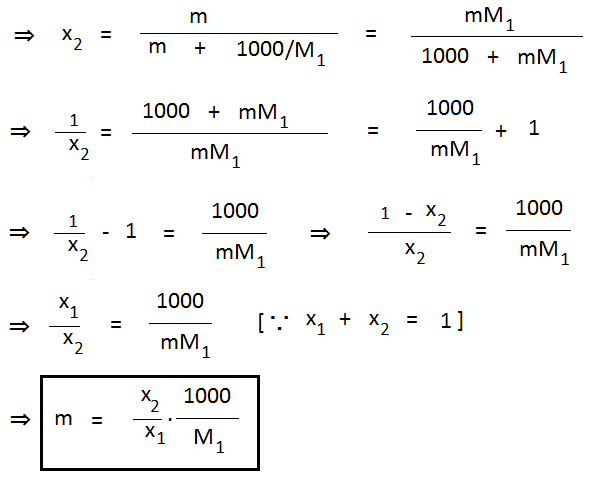








Comments (No)