Determination of Rate Law, Rate Constant and Order of Reaction:
Different experimental methods used for the determination of the rate law, rate constant and order of reaction are briefly described below-
(1) Graphical Method- This method can be used when there is only one type of reactant. It is hit and trial method. Example- Consider the reaction, nA ——> Products.
| Rate of reaction = k [A]n [where ‘n’ is the order of reaction] |
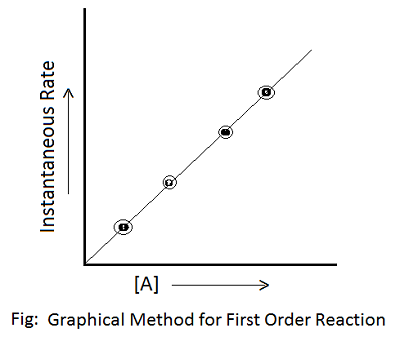
Stepwise Procedure for the Graphical Method:
- Concentration of reactant is measured at different time intervals by employing a suitable method.
- The graph is plotted between concentration and time and then, tangents are drawn to the curve at different times to get an instantaneous rate of reaction.
- Again a graph is plotted between instantaneous rate and the concentration of reactant with its different powers, i.e., [A], [A]2, [A]3, ……….. so that a straight line is obtained. The power of the concentration w.r.t. which the instantaneous rate gives the straight line is the order of the reaction.
(2) Initial Rate Reaction or Oswald’s Isolation Method- This method can be used irrespective of the number of reactants (whether same or different) involved. Example- Consider the reaction, n1A + n2B + n3C ——> Products.
This method consists in finding the initial rate of reaction taking known concentrations of the different reactants (A, B and C). Now the concentration of one of the reactants is changed (say A) taking the concentration of other reactants (B and C) same as before. The initial rate of reaction is determined again. This gives the rate of reaction w.r.t. ‘A’ and hence the order of reaction w.r.t. ‘A’. Now the same experiment is repeated with other reactants ‘B’ and ‘C’ and the order of reaction w.r.t. ‘B’ and ‘C’ is determined. Combining the different rate expressions, the overall rate expression and hence the order of reaction is determined. Example- If the order w.r.t. ‘A’, ‘B’ and ‘C’ are α, β, γ, then
| Rate = k [A]α [B]β [C]γ |
So, the overall order of reaction is ( α + β + γ).
(3) Integrated Rate Law Method- This is the most common method for studying the kinetics of a chemical reaction. Example- Consider the reaction, nA ——-> Products.
If we start with ‘a’ mole/litre of ‘A’ and in time ‘t’ sec, ‘x’ moles /litre have reacted so that the concentration after time ‘t’ is (a – x) moles/litre. Then the rate of reaction (if it is Ist order) is dx/dt = k (a – x) and dx/dt = k (a – x)2 for 2nd order and so on. These equations are differential equations and can be integrated, which are very convenient as they contain the concentration of a reactant at different times and hence can be solved to get the value of ‘k’ from the data. However, it is also a hit and trial method. The results of the experiment are substituted in the expressions for the rate constant of different orders one by one. The expression which gives a constant value of the rate constant (k), decides the order of reactions.
(4) From Half-Life Method- The half-life period depends differently on the initial concentration of the reactants [R]0 for a different order of reaction, we have-
| For Zero Order Reaction, t1/2 ∝ [R]0 For First Order Reaction, t1/2 is independent of [R]0 For Second Order Reaction, t1/2 ∝ 1/[R]0 For nth Order Reaction, t1/2 ∝ 1/[R]0n-1 |
From the variation of t1/2 with [R]0, it becomes easy to find the order of the reaction, i.e. starting with two different initial concentrations {[R]0}1 and {[R]0}2 and finding their half-lives, the order of reaction ‘n’ can be calculated as-
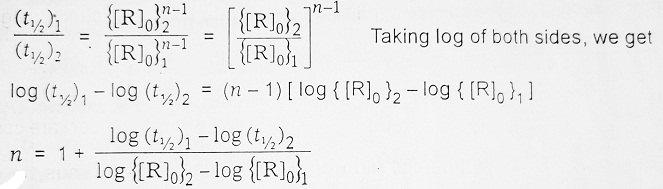








Comments (No)