Factors Affecting the Rate of Reaction:
The rate of any particular reaction depends upon the following factors-
- Concentration of Reactants- The rate of reaction depends upon the concentration of the reactants. This is clear from the Law of mass action, according to which the rate of reaction is directly proportional to the active mass or molar concentration of reactants. For example- The rate of burning of wood depends upon the concentration of oxygen. A piece of wood burns slowly in the air (which contains about 20% of oxygen) but burns rapidly in pure oxygen because the concentration of oxygen in air is less than in pure oxygen.
- Nature of Reactants and Products- The chemical reaction involves the breaking of old bonds and the formation of new bonds. Thus, the reactivity of a reactant in a chemical reaction is related to the ease with which the specific bonds are broken or formed. So, a chemical reaction involving a solid substance in the coarse form will be rapid to the reaction of the same solid in bulk form and vice-versa.
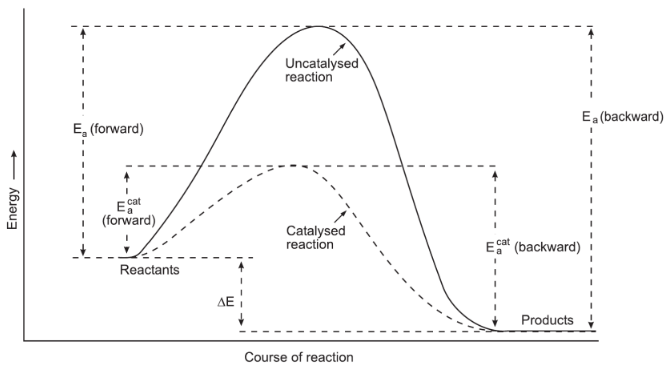
- Presence of a Catalyst- A catalyst is a substance, which influences the rate of the reaction without itself undergoing any chemical change. The effect of catalyst on the rate of reaction is explained on the basis of the activated complex theory. According to this theory, every reaction has the minimum energy required to convert reactants to products called activation energy. But when a catalyst is used, it takes the reaction through an alternate path which has either more or less activation energy than uncatalyst reaction as shown in the figure. So, the rate of reaction is affected.
- Exposure to Radiation- All photochemical reactions are reactions of zero order and are influenced by radiations. Since these reactions follow a chain mechanism which requires the free radicals for the initiation of chains. The photons of light produce the free radicals in such reactions and hence the rate of reaction depends upon exposure to radiation.
- Dependence of Reaction Rates on Temperature- Temperature has a great influence on reaction rates. In general, an increase in temperature increases the rate of the reaction and vice-versa. General approximate rules state that the rate of reaction becomes double for every 10° rise in temperature. This is also called temperature co-efficient and is the ratio of rate constant of reaction at two temperatures differing by 10°C, i.e.,
| Temperature co-efficient = Rate constant (T + 10°)C/Rate constant at T°C |









Comments (No)