Activation Energy and Activated Complex:
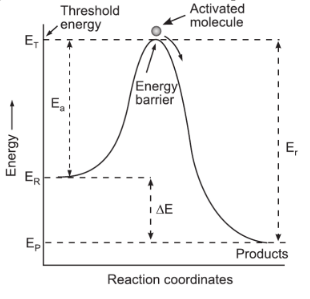
The excess of energy (over and above the average energy of the reactant), which must be supplied to the reactants to undergo chemical reactions, is called activation energy (Ea) and is the difference between the threshold energy needed for the reaction to the average kinetic energy of all the reacting molecules, i.e.
| Activation Energy = Threshold Energy – Average Kinetic Energy of the Reacting Molecules |
Thus, each reaction has a definite value of Ea.
If Ea is low, more of reactant molecules convert into products and hence fast is reaction and vice-versa.
Thus, when the colliding molecules possess the kinetic energy equal to Ea, the atomic configuration of species formed at this stage is different from reactants or products. This stage is called the activated state or transition state and such a configuration of molecules is called activated complexes which have the old bonds as well as new bonds to be formed. But all the bonds are weak. For example- in the case of the following reactions-
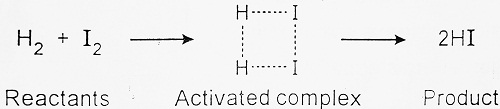
Thus, the reaction involves some energy barrier, which must be overcome before products are formed. This energy barrier is called activation energy as shown below-
Kinetic Stability of Fuels:
The unusual stability of fuels even in the presence of oxygen or air can now be explained on the basis of the concept of activation energy. In general, the activation energy of combustion reactions is quite high. When a flame is applied to the mixture of fuel and air, the contents near the flame are raised to a high temperature. The supplied energy is enough to cause the combustion of a portion of the fuel in oxygen. The heat liberated by the combustion increases the temperature of the remaining fuel and the flame once produced is sustained. Thus we realize that many reactions which are feasible from the consideration of free energy may be prevented from occurring due to the high activation energy barrier. Such energy barriers are blissful for the existence of life on earth because they provide stability to the reactants like fuels, contents of explosives in bombs, etc., which otherwise are unstable from the point of view of thermodynamics. This leads us to make a concluding statement that the reactants which are thermodynamically unstable at ordinary temperature may not be kinetically unstable.








Comments (No)