Table of Contents
Preparation of Lyophobic Sols:
These sols can be prepared by the following methods-
Condensation or Aggregation Methods:
This method is based on the principle that the particles of atomic or molecular size are allowed to condense or aggregate to give rise to the formation of colloidal sized particles. These methods are of two types-
Chemical Methods:
The various chemical methods used are as follows-
- Double Decomposition- This method is used for preparing the colloidal sols of insoluble substances. For example- an orange coloured colloidal sol of Arsenious Sulfide (As2S3) is obtained by passing a current of H2S gas through a boiling solution of Arsenious Oxide-
| As2O3 + 3H2S ———-> As2S3 + 3H2O |
- Oxidation Method- This method is used for preparing colloidal sols of non-metallic substances like S, P4, I2 etc. by the oxidation of their water-soluble salts. For example- a milky colloidal sol of sulfur is obtained by passing H2S gas through oxidising agents like Br2 water or dilute HNO3.
| H2S + Br2 ———> S + 2HBr 3H2S + 2HNO3 ———–> 3S + 2NO + 4H2O |
- Reduction Method- This method is used for the preparation of colloidal sols of metals like Ag, Au, Sn, Pb, Pt etc. by the reduction of their suitable water-soluble salts. For example- a purple colloidal sol of gold is prepared by reducing an aqueous solution of Gold Chloride (AuCl3) with stannous chloride (SnCl2).
| 2AuCl3 + 3SnCl2 ————-> 2Au + 3SnCl4 |
- Hydrolysis Method- This method is used for the preparation of colloidal sols of hydroxides of weakly electropositive metals like Fe, Al, Sn etc. For example- a reddish-brown colloidal sol of Ferric hydroxide is obtained by adding a very dilute solution of FeCl3 (3-4%) in boiling water with constant shaking.
| FeCl3 + 3H2O ⇌ Fe(OH)3 + 3HCl |
Physical Methods:
The various physical methods used are as follows-
- By exchange of solvent- When a true solution is mixed with an excess of the other solvent in which the solute is insoluble but solvent is soluble, a colloidal solution is obtained. For example- when a solution of sulfur in alcohol is poured in excess of water, a colloidal solution of sulfur is obtained.
- By Change of Physical State- Colloidal sols of substances like S, Hg, I2 etc. are obtained by passing their vapours into cold water containing NH4Cl as a stabilizer.
- By Excessive Cooling- The colloidal solution of ice in an organic solvent such as CHCl3 or ether can be obtained by freezing a solution of water in the solvent. The molecules of water which can no longer be held in solution separately combine to form particles of colloidal size.
Dispersion Methods:
In these methods, large particles of the substance are broken into particles of colloidal dimensions in the presence of a dispersion medium. Since the sols formed are highly unstable. They are stabilized by adding some suitable stabilizer. Some of the methods employed for carrying out dispersion are given as follows-
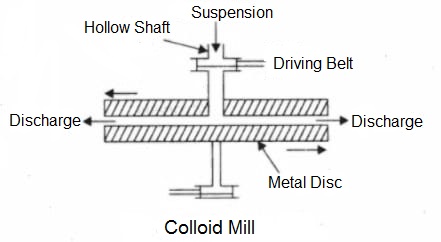
- Mechanical Dispersion- This method is used for preparing ointments, dental creams, writing ink, paints etc. which are of colloidal nature. The substance to be obtained in the colloidal state is finely powdered and then made into suspension by shaking with a suitable dispersion medium. The suspension is then introduced into a simple colloidal mill which consists of two metal discs nearly touching each other and rotating at a very high speed (7000 revolutions per minute) in opposite directions when the suspension particles are broken into colloidal sized particles. A small quantity of gum is added as a stabilizer.
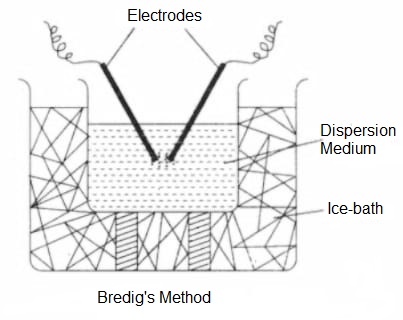
- Electrical Dispersion Method (Bredig’s arc method)- This method is used for preparing colloidal sols of metals like Au, Ag, Sn, Pb, Pt etc. The two rods of metal to be obtained in the colloidal state are made electrodes that are dipped in the dispersion medium (water) kept cooled by surrounding it with ice. When an electric arc is struck between the electrodes, the metal electrodes vaporizes due to intense heat. The vapours of the metal undergo condensation in ice-cold water to form a colloidal sol, some of the vapours may undergo excessive cooling due to which bigger particles may be formed in the sol which is removed by filtration. The sol thus obtained is stabilized by adding a little NaOH (alkali) in water.
- Peptization- It is the process of converting a fresh precipitate (which is simply a cluster of particles of colloidal size held by weak forces) into a colloidal sol by the addition of an electrolyte known as a peptizing agent. It involves the adsorption of similar ions from the electrolyte by fine particles of the precipitate which develops a positive or negative charge on it and ultimately it breaks up into smaller particles having colloidal dimensions. For example- a reddish-brown colloidal sol of Fe(OH)3 is obtained by adding a small amount of FeCl3 (Electrolyte) to freshly precipitated ferric hydroxide.
| FeCl3 ⇌ Fe3+ + 3Cl– (Ionisation) Fe(OH)3 (precipitated) + Fe3+ (From Electrolyte) ——–> [Fe(OH)3]Fe3+ (Reddish Brown Sol) |





![Water Cycle In Nature [Hydrological Cycle] 11 water cycle in environment](https://gkscientist.com/wp-content/uploads/2020/09/water-cycle-in-environment-350x200.jpg)


Comments (No)