Table of Contents
Brownian Movement and Tyndall Effect:
Brownian Movement:
When a colloidal sol is observed under an ultramicroscope, the dispersed phase particles were seen to be moving continuously in zig-zag paths. This continuous and random motion of colloidal particles in the dispersion medium is known as the Brownian movement. It was first noticed by Sir Robert Brown (1827) in the case of pollen grains suspended in water. The Brownian movement has been found out to be a characteristic of all colloidal sols irrespective of their nature.
Cause of Brownian Movement:
The cause of Brownian movement is the impacts of dispersion medium particles with the colloidal particles. A colloidal particle in the dispersion medium is surrounded by a large number of particles of the dispersion medium which constantly bombard it from all directions. As a result of this unequal bombardment, a large momentum is imparted to the colloidal particle which is then knocked out in a zig-zag manner and thus exhibits Brownian movement. The Brownian movement goes on decreasing with the increase in particle size because the chances of unequal bombardment decreases. Thus, suspensions do not shown any such movement due to a bigger particle size.
Significance of Brownian Movement:
- Brownian movement provides a direct demonstration of the ceaseless motion of molecules as postulated by kinetic theory.
- Brownian movement is said to be responsible for the stability of colloidal sols as it counteracts the force of gravity acting on the colloidal particles and does not allow them to settle down.
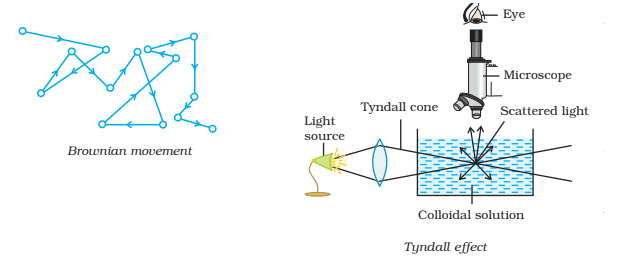
Tyndall Effect:
John Tyndall (1869) observed that when a strong beam of light is concentrated on a colloidal sol placed in a dark room, the path of the beam becomes illuminated by a bluish coloured light when seen in a direction at right angles to that of the incident light. This phenomenon is known as the Tyndall Effect and the illuminated path is known as Tyndall Cone.
Cause of Tyndall Effect:
The cause of the Tyndall effect is the scattering of light by colloidal particles. The colloidal particles first absorb light and then a part of this light is scattered from the surface of a colloidal particle in a colloidal sol since the intensity of scattered light is maximum in the direction at right angles to the incident light, the path of light becomes visible only when seen in that direction. The true solution particles being smaller in size can not scatter the absorbed light and thus the field of vision remains dark when a strong beam of light is concentrated upon them. Thus, the Tyndall effect helps in differentiating a true solution from a colloidal sol.
Some examples of Tyndall Effect are-
- Blue colour of sky and sea water.
- Visibility of tails of comets and twinkling of stars.
- Visibility of projector path and circus light.
- Visibility of sharp ray of sunlight passing through a slit in dark room.









Comments (No)