Table of Contents
Quantum Yield:
It may be defined as the number of molecules of reactants consumed or products formed per quantum of light absorbed.
| i.e. Φ = No. of molecules of reactants consumed or products formed in the given time/no. of quanta of light absorbed in same time or Φ = No. of moles of reactants consumed or products formed in a given time/no. of Einsteins of light absorbed in same time or Φ = Rate of chemical reaction/No. of Einsteins of light absorbed |
The photo efficiency of the reactions differs widely. The integer value 1 is found only for a very limited number of simple reactions otherwise the value of Φ may vary from zero to 106. For example,

Reasons for High Quantum Yield:
The main reasons for high quantum yield are:
(1) The primary process might generate free radicals which are capable of generating chain reactions example, in the combination of H2 and Cl2 to give HCl, when the quantum yield is of the order of 106.
(2) If the secondary reaction is exothermic, the heat of the reaction may activate other molecules, causing them to react, resulting in a high quantum yield.
(3) Reactions are subsequent to the primary reaction, for example, one photon absorbed in the primary reaction dissociates one molecule of the reactant. The excited atom may start subsequently a secondary reaction in which further molecule is decomposed.
| AB + hν ———-> A + B (Primary) AB + A ———-> A2 + B (Secondary) |
Hence one photon of radiation has decomposed two molecules one in the primary reaction and one in the secondary reaction. Hence quantum yield is 2.
(4) Intermediate gets formed and acts as a catalyst.
Reasons for Low Quantum Yield:
The main reasons for low quantum yield are:
(1) Deactivation of reacting molecule- The excited molecules in the primary process may be deactivated before they get an opportunity to react. This is caused by collision with some inert molecule or by fluorescence.
| A + hν ———-> A* (Activation) A* ———-> A + hν’ (Fluorescence) |
(2) Occurrence of reverse primary reaction- Primary reaction generally yields a polymer. The product then undergoes a thermal reaction giving back the reactant molecules.
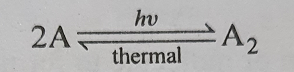
Reverse thermal reaction proceed till equilibrium.
(3) Recombination of dissociated fragments- In the primary process the reactant molecule may dissociate to give small fragments. These fragments can recombine to give back the reactant.
| AB + hν ———-> A + B A + B ———-> AB |
Thus the secondary reactions involving the fragments to form a product will not occur. This will greatly lower the yield.









Comments (No)