Intergranular Corrosion:
Stainless steel can pose special corrosion challenges since its passivating behavior depends on the presence of a minor alloying component (Chromium, typically only 18%). Due to the elevated temperature during welding or during improper heat treatment, there is the formation of chromium carbides in the grain boundaries of stainless alloys. This chemical reaction attacks the material of chromium in the zone near the grain boundary making these areas much less resistant to corrosion. This creates a galvanic couple with the well-protected nearby cathode (alloy), which leads to weld decay (corrosion of the grain boundaries near welds) in highly corrosive environments. Special alloys, either with low carbon content or with added carbon getters such as titanium and niobium, can prevent this effect, but the latter require special heat treatment after welding to prevent a similar knifeline attack. As its name applies, this is limited to a small zone, often only a few micrometers across. This zone is very near the weld, making it even less noticeable.
Intergranular Corrosion is a type of localized corrosion. In this type of corrosion, the grain boundaries are more sensitive i.e., maximum corrosion occurs at grain boundaries because this part acts as an anode. Whereas, grain centres remain unattached and this area is said to be a cathodic area where corrosion does not take place.
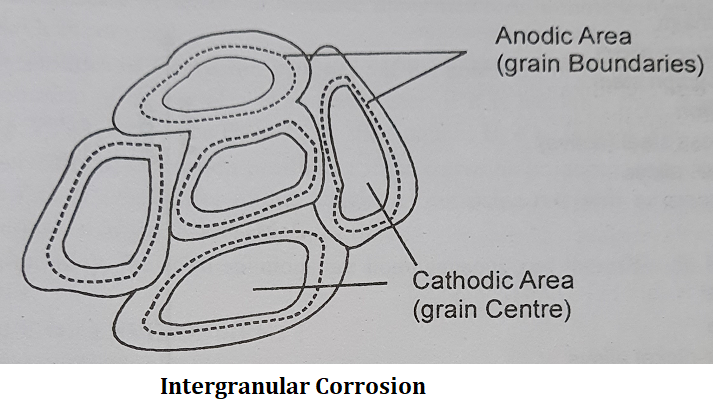
| Note: Corrosion may be classified on the basis of the area of metal on which corrosion takes place. According to this the two main types of corrosion are: (1) Uniform Corrosion- It results from the sites, that are distributed over a metal surface where the anodic and cathodic reactions take place. Uniform corrosion damage, sometimes called wastage, is usually manifested in the progressive thinnings of a metal par until it virtually dissolves away or becomes a delicate lace-like structure. (2) Localized Corrosion- Localized corrosion results from the sites, necessarily fixed in location, and are distributed over a metal surface where only the anodic reactions take place. It is localized to particular areas (anodic area). It is not effective to the entire surface of the metal. |








Comments (No)