Silicates:
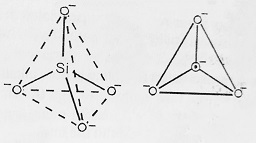
Silicates form an important class of minerals that constitute rocks and soil. Example- micas, clays, feldspars, asbestos etc. According to X-ray crystallographic studies, all silicates have SiO44- units formed through SP3 hybridization and thus have tetrahedral structure as shown below-
The electronic structure of SiO44- ion is written as-
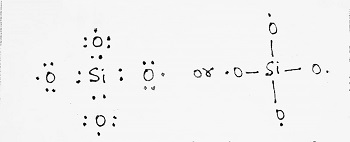
This structure shows that the octet of Si is complete while each of the four O-atoms is short of one electron and have a tendency to get electrons from some other atoms and so acquire negative charges forming discrete SiO44- ions. Example- Zircon (ZrSiO4) and Wilemite (Zn2SiO4).
Classification of Silicates:
Silicates differ from one another in the manner in which SiO44- tetrahedra are joined together. The silicates are classified into the following different types depending upon the number of corners (0, 1, 2, 3, or 4) of the SiO44- tetrahedron shared with the other tetrahedra.
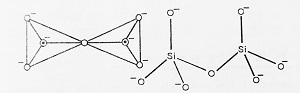
- Orthosilicates- These are simple silicates in which SiO44- tetrahedra do not share corners (O-atoms) with one another and exist as discrete SiO44- orthosilicate anions as shown in the figure. Example- Zircon (ZrSiO4), Forestrite (Mg2SiO4), Zn2SiO4. In these compounds, SiO44- ion is linked to M2+ ions through a coordinate bond.

- Pyrosilicates or Islands- When two SiO44- tetrahedra join through a single common oxygen atom (share a corner), Island structures having the formula Si2O76- are obtained known as Pyrosilicates. Example- Thortveitite i.e. Sc2(Si2O7), Hemimorphite i.e. Zn3 (Si2O7) Zn(OH)2.H2O.
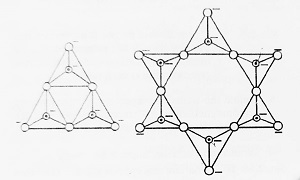
- Cyclic or Ring Silicates- These are those silicates in which two O-atoms of each tetrahedron are shared with others to form closed rings that starts with general formula (SinO3n)2n-. Example- Wollastonite (Ca3Si3O9), Beryl (Be3Al2Si6O18).
- Chain Silicates- If two O-atoms per SiO44- tetrahedron are shared such that a linear single strand chain of the general formula (SinO3n)2- is formed known as chain silicates. Thus, the primary unit of this chain structure is SiO32-. Silicates of this type are called Pyroxenes or metasilicates. Example- Enstatite (MgSiO3), Diopside [CaMg(SiO3)2], Spodumene [LiAl(SiO3)2]. Two chains may be linked together to form double chains, thereby having the formula (Si4O11)n6n- known as Amphiboles which are present in asbestos.
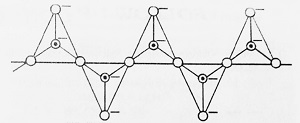
- Sheet Silicates- The sharing of three corners (i.e. three oxygen of each tetrahedron) result in an infinite two-dimensional sheet structure of the formula (Si2O5)n2n- known as Sheet Silicates. Example- Clay, Mica etc.
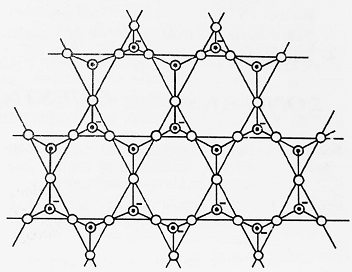
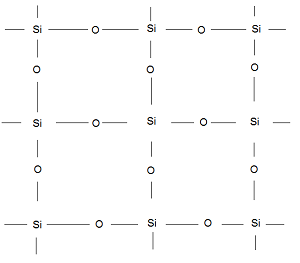
- Three-dimensional Silicates- These are those silicates in which all the four O-atoms of SiO44- tetrahedron are shared with other tetrahedra resulting in a three-dimensional network structure. The empirical formula for this type of silicate is (SiO2)n. Example- Quartz (Silica).








Comments (No)