Table of Contents
What is Polymer?
The process of joining together of a large number of simple molecules by covalent bonds to form long chains of a macromolecule of high molecular mass is known as Polymerisation and the macromolecules so formed is known as Polymer (Poly- many; Mer- unit). The small molecules which constitute the repeating units in a polymer are called Monomers. Example- Polyethylene is a polymer obtained by the Polymerisation of ethylene monomers i.e.
The number of monomer units in the chain is called Degree of Polymerisation.
Polymers with a low degree of Polymerisation are called Oligomers and with a high degree of Polymerisation are called High Polymers.
Homopolymers- Polymers formed from one kind of monomers are called Homopolymers. Example- Polyethylene, Polypropylene, Polyvinyl chloride etc.
Copolymer or Mixed Polymers- Polymers formed from more than one kind of Monomer units are called copolymers or mixed polymers. Example- Buna-s rubber which is formed from 1,3-Butadiene and Styrene; Bakelite which is formed from Phenol and Formaldehyde.
Structure of Polymers:
Linear Polymers- In these polymers, the monomeric units are linked together to form long chains (straight) which are stacked over one another to give a well-packed structure and thus have high densities, high tensile strength and high melting points. Example- Polyethylene, Nylons and Polyesters etc.

Branched Chain Polymers- In these polymers, the monomeric units are linked to constitute long chains having branches. Branched chain polymers are irregularly packed and thus have low density, lower tensile strength and lower melting points than linear polymers. Example- Amylopectin, Glycogens etc.

Cross Linked Polymers- In these Polymers, the linear Polymer chains are joined together to form a three-dimensional network structure. These are hard, rigid and brittle. Example- Bakelite, Melamine Formaldehyde resin etc.

Classification of Polymers:
Polymers are broadly classified into two classes- natural polymers and synthetic polymers.
Natural Polymers:
These are naturally occurring polymeric substances occurring mostly in pants and animals. Example- starch, cellulose, rubber, nucleic acids, proteins etc.
Synthetic Polymers:
These are man-made polymeric substances that are synthesized in the laboratory. These are long-chain organic molecules containing thousands of monomeric units. Example- Polythene, Polystyrene, PVC, Teflon, Nylon, Bakelite, Dacron, Glyptal etc. These are further classified into two classes- addition polymers and condensation polymers.
Addition Polymers:
When a large number of monomer units join together to form long chains without the elimination of any by-product molecules, the product formed is Addition Polymer and the process involved is Addition Polymerisation. The monomer units are unsaturated compounds and are generally the derivatives of Alkenes. Example-

Condensation Polymers:
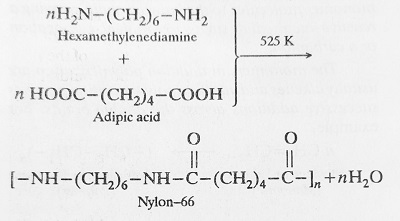
When monomers containing some active functional group (same or different) combine together with the elimination of simple molecules (like H2O, HCl, ROH, NH3 etc.), the product formed is called Condensation Polymer and the process involved is called Condensation Polymerisation. Example- Nylon-66 is a condensation polymer of Hexamethylenediamine and Adipic acid.









Comments (No)