Entropy and Enthalpy in Living Systems:
(1) Entropy in Living Systems- According to the second law of thermodynamics, any system when left to itself tends to increase entropy or disorder. The term entropy was used by Rudolf Clasius in 1851. Literally, it means a change within. Dissipation into the environment is the ultimate fate of all energy. Entropy and dissipation are due to the tendency of energy to be evenly distributed.
Energy gets transferred from high-energy areas to low-energy areas. During this movement, there is a collision of particles leading to disorder. This disorder is called entropy. Due to the collision of particles, heat is generated and dissipated in the surroundings causing a loss of some energy.
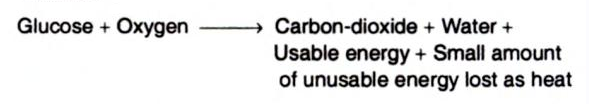
Example: Entropy can be illustrated by the example of glucose oxidation. Aerobic organisms extract free energy from glucose obtained from their surroundings. To obtain energy, they oxidize glucose in the presence of oxygen (O2) and produce CO2 and H2O. In this process, the surrounding environment undergoes an increase in entropy whereas the organism remains in a steady state. Some of the entropy also arises from the dissipation of heat. Further, when a solid substance like glucose is changed to a liquid or gaseous product, the molecules have more freedom to move or fill spaces as compared to a solid and there is an increase in molecular disorder and thus an increase in entropy.

In the above reaction, there is an increase in the number of molecules leading to an increase in molecular disorder thus leading to entropy.
(2) What is Enthalpy and Free Energy? In a biological system, the total energy including usable energy that can do work and unusable energy that is lost to disorder is called enthalpy. The amount of usable energy that is available for doing work is called free energy.
The energy required to destabilize chemical bonds and to initiate a chemical reaction is called activation energy. Reactions that occur without outside intervention, release free energy, and can perform work, are called spontaneous reactions.
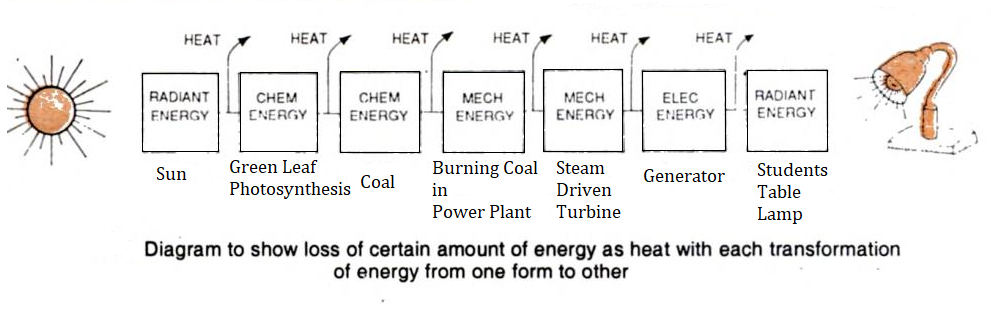
Thus every energy transfer and transformation reaction in the system causes a steady loss of energy leading to increased entropy and decreased energy content of the system (enthalpy). It means that if an active chemical system is capable of restoring and recouping energy (the entropy), it establishes an equilibrium and survives, otherwise it gradually finishes (for example dying of stars).
Protoplasm, the living substance is the most active chemical system of nature with maximum energy conversion, yet it maintains its organization (enthalpy) by continuously obtaining free energy from the environment. It is this capability which clearly distinguishes protoplasm from non-living systems. Living systems recoup enthalpy either by trapping solar energy in chemical bonds of glucose synthesized by autotrophic green plants or in the form of food.









Comments (No)