Table of Contents
Enzymes And Their Properties:
What is Enzymes?
Enzymes are a major class of proteins. They are also called as biological catalysts (biocatalysts) because they speed up the rate of reaction in organisms. In our body, numerous biological reactions are occurring which are not possible without enzymes.
The enzymes have been defined as the simple or combined proteins acting as specific catalysts. They are soluble colloidal molecules produced by living cells. All enzymes are globular proteins. They have a complex 3’D’ structure. They can bind with the substrate molecule to a part of their surface. The enzymes do not change the chemical reaction but only speed up the rate of reaction. For example- carbonic anhydrase accelerate the dissolution of CO2 in blood. The dissolved CO2 is transported to the lungs. Then it is expired at a rate greater than 106 times, as compared to the reaction without this enzyme. Catalytic power of an enzyme is measured by the turnover number or molecular activities. Turnover number is defined as the number of substrate molecule converted into product per unit time, when an enzyme is fully saturated with substrate. Turnover of each enzyme differ. Carbonic anhydrase has the highest turnover number of 6 X 106 sec-1. Usually, the turnover number falls between 1 and 104 sec-1. Suppose a substrate reacts in the presence of an enzyme and produces a product (P), it may be written as below-
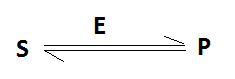
Important Properties or Characteristics of Enzymes:
- Enzymes are polymeric organic compounds with high molecular weight.
- Enzymes are wholly (example- Amylase, Trypsin and Pepsin) or partially (example- Hexokinase, ATPase, Catalase, Succinate DHase) proteinous in chemical composition except Ribozymes and RNase-P are two non-proteinous enzymes in which RNA acts as a catalyst.
- They increase the rate of a chemical reaction and bring about equilibrium very soon.
- The enzymes are specific in their action. For example- enzyme sucrase acts upon raffinose and gives rise to sucrose and lactose.
- Enzymes act as a biocatalyst and they are not used up during the chemical reaction. However, they increase the rate of a chemical reaction.
- All enzymes controlled reactions are reversible depending upon the availability of reactants, end products, pH and energy requirements.
- Enzymes are temperature specific. Each enzyme is most active at a specific temperature called optimum temperature. Example- 37°C for amylase enzyme. Range of optimum temperature is 25-40°C.
- The enzymes show maximum activity at an optimum pH. For example- enzyme pepsin acts at pH 2.0 whereas trypsin at pH 8.5.
- Some enzymes are intracellular or endoenzymes (act within the same cell). Example- respiratory enzymes of mitochondria but some enzymes are extracellular or exoenzymes. Example– digestive enzymes of gastric glands, salivary glands and pancreas.
- The extremely small amount of enzyme is required to bring about measurable change.
- In metabolic pathways, enzymes act in a specific sequence.
- They are very unstable compounds mostly soluble in water, dilute glycerol, sodium chloride solution, and in dilute alcohol as well.
- Besides breaking down, enzymes are capable of rebuilding specific elements out of the final products either by reversible synthesis or else by specific synthesis by specific enzymes.








Comments (No)