Table of Contents
Intermolecular Forces:
The forces present between the molecules of a substance are known as intermolecular forces. Such forces exist in the three states of matter and are responsible for many structural features and physical properties of matter. In addition, these are intermolecular forces that exist within each molecule, influence the chemical properties of the substance. These forces are weak forces. These forces arise due to the following type of interactions-
Dipole-dipole interaction (or Keesom Forces):
These type of forces occur in molecules which have permanent electrical dipoles such as HCl, NH3, H2O etc. These forces arise due to electrostatic interactions between oppositely charged ends of the polar molecules. This interaction was first studied by Keesom in 1912 and called Keesom forces. These forces produce a net attractive influence. The interaction energy is found to be inversely proportional to the sixth power of the distance between molecules, i.e.,
Interaction Energy ∝ 1/r6 = -K 1/r6
where ‘K‘ is a constant of proportionality and a negative sign is used to indicate the forces of attraction. Example- H-bonding in different molecules.
Ion-dipole interaction:
Ion-dipole interactions are between an ion (either cation or anion) and a polar molecule. The strength of these forces depends upon-
- Charge and size of the ion- It may be noted that in the case of cations and anions have the same charge, cations interact more strongly as compared to anion due to its smaller size. It is according to Fajyan’s rule.
- The dipole movement and the size of the polar molecules- Example of such interaction is hydration of different ion. Example- Hydration of Na+ ion is more than hydration of Cl– ion due to ion-dipole interaction.
Ion-induced Dipolar Interactions:
Such dipole interactions occur between ions and non-polar molecules. In such cases, ions induce polarity on the polar molecule when they approach each other. Example- the interaction between NO3– ion and molecule of I2. As they approach each other the negatively charged NO3– ion induces the polarity on the I2 molecule which also becomes dipolar.
Instantaneous Dipolar Interaction:
In the molecular solids, the molecules are closely packed in space. If they are polar, then binding forces are either dipole interaction or Hydrogen bonding. However, if they are non-polar in nature (H2, O2, Cl2, CH4, etc.). Then binding forces are Vander Wall’s Forces. These forces are also present in noble gases.
These forces of attraction were first proposed by Dutch scientist J.D.Vander Wall. It can be understood by taking an example of noble gases like He. In case of Helium, the most favourable arrangement is that when a nuclear charge is present in the centre and the two electrons are located diametrically opposite for each other, so that centres of positive or negative charge coincide. Thus, He-atom is completely non-polar as shown in fig.
However, electrons do not have fixed positions. It is also possible that at the particular instant the electronic distribution may not remain symmetrical and this atom becomes instantaneous dipolar as shown in fig.
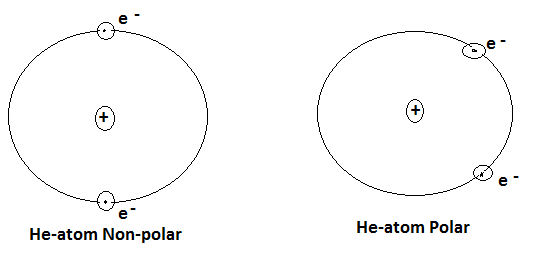
Though the atom will immediately become symmetrical. But it will induce polarity on the neighbouring atoms. Two such atoms are attracted towards each other by attractive forces known as Vander Wall’s Forces.









Comments (No)