Table of Contents
Jablonski Diagram:
When radiation of suitable energy is absorbed by the molecule, then an electronic excitation takes place.
Let the electron get excited from ground state S0 to singlet excited state S2. S2 state is highly unstable and has a lifetime of only 10-11 sec, so it decays into another excited state S1 by Radiationless Decay (RD). This is called internal conversion (IC). S1 state is comparatively long-lived (10-8 sec), and is very important in photochemistry.
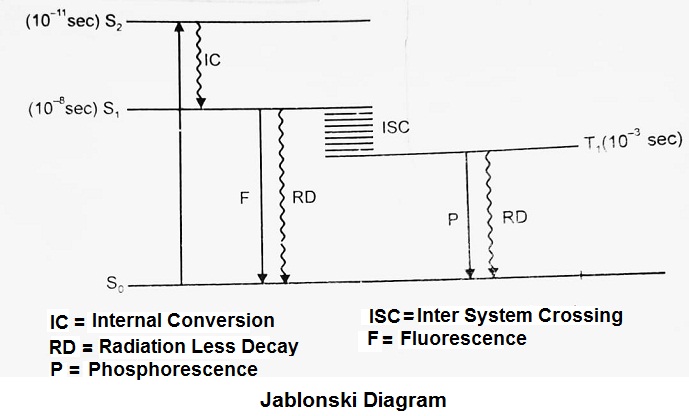
At this stage, there are four possibilities:
- The electron may jump to ground state with instant emission of photon. This is called as Fluorescence (F).
- It may return to ground state S0 by radiationless decay (RD) through shuffling of energy in vibrational moulds (energy levels).
- It may undergo chemical reaction.
- It is quite possible that before the occurrence of any of the above three processes, the electron may go to triplet excited state. This is called as Inter Ssytem Crossing (ISC). According to spectroscopic rules the transitions with change in multiplicity are forbidden. The reverse is never possible i.e. T1 to S1 conversion is not possible.
At T1 again there are the following three possibilities:
- A chemical reaction may take place.
- The electron may return to ground state S0 by RD (radiationless dacay).
- The electron may jump to ground state with the emission of a photon. This is called as phosphorescence (Delayed Fluorescence is called Phosphorescence).
Explanation of Fluorescence and Phosphorescence on the Basis of Jablonski Diagram:
Fluorescence:
The instantaneous emission of radiation due to transition between two states of the same multiplicity i.e. from singlet excited state S1 to singlet ground state S0 is called Fluorescence and Substances are called Fluorescent substances. The emitted radiation in fluorescence has a lower frequency than incident radiation.
Examples- (a) Organic dyes like Eosin, fluorescein etc. (b) Compounds like chlorophyll.
Resonance Fluorescence- In contrast to molecules, atoms emit radiations of exactly the same frequency as the frequency of incident radiation. It is called Resonance Fluorescence.
For molecules ν ≠ ν’ but for atoms ν = ν’.
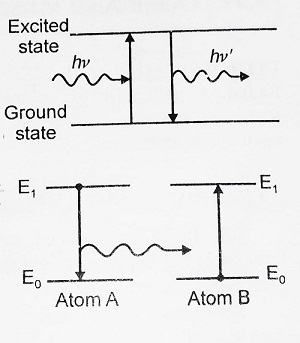
Quenching of Fluorescence- Sometimes a photochemical excited atom collides with another atom and transfers its energy. This results in diminishing the intensity of fluorescent radiation. This process is called Quenching of Fluorescence.
Applications of Fluorescence:
- To detect leaks by adding it to water system.
- Optical brightners are added to detergents to brighten the fabrics.
- Fluorescent tubes are used for lightning purpose.
- Fluorescent microscopes and Fluorescopes are used in X-ray diagnosis for testing conditions of food stuffs and detection of ringworms.
Phosphorescence:
It is the emission of radiation between two states of different multiplicities i.e., from triplet excited state T1 to singlet ground state S0. According to selection rules, this transition is not allowed because of the spin of an electron changes in this transition.
Fluorescence stops as soon as the external light is cut off. But certain substances continue to glow for some time even after the source of light is removed. Thus the phenomenon of delayed fluorescence is called Phosphorescence and such substances are called phosphorescent substances or phosphor. For example Zinc Sulfide (ZnS).
Difference Between Fluorescence and Phosphorescence:
The following are the points of difference between Fluorescence and Phosphorescence.
| Fluorescence | Phosphorescence |
|---|---|
| It is the radiation emitted when the transition takes place between two states of the same multiplicity. | It is the radiation emitted when the transition takes place between two states of different multiplicity. |
| It is a rapid process. The decay time is 10-3 to 10-8 sec. | It is a slow process. The Decay time is 10-4 to 150 sec. |
| It is an allowed process. | It is a forbidden process. |
| It can be observed in solution at room temperature. | It is not observed in solutions at room temperature. |








Comments (No)