Phase Diagram of Carbon Dioxide:
The phase diagram for the carbon dioxide (CO2) system resembles the water system because it has also three areas for solid, liquid, and gaseous phases. The phase diagram for the CO2 system is shown below.
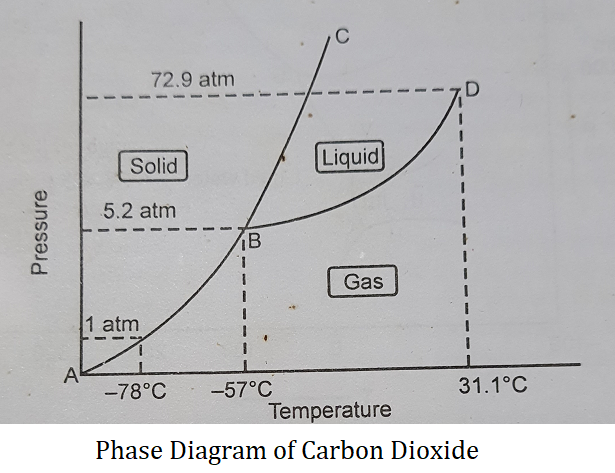
The phase diagram for the CO2 system has the following characteristic:
(1) Curves- Like a water system, along each curve in the CO2 system, two phases are in equilibrium with each other, As C = 1, therefore the degree of freedom is one as shown below.
| F = C – P + 2 F = 1 – 2 + 2 F = 1 Therefore the curves are univariant. |
The curve AB is the sublimation curve. Along this curve, solid CO2 is in equilibrium with CO2 gas.
| CO2 (s) ⇌ CO2 (g) |
The curve BD is the vaporization curve. Along this curve, liquid CO2 is in equilibrium with CO2 gas.
| CO2 (l) ⇌ CO2 (g) |
The curve BC is the fusion curve. Along this curve, solid CO2 is in equilibrium with liquid CO2.
| CO2 (s) ⇌ CO2 (l) |
(2) Areas- In the areas only one of the three phases is present. As C = 1 therefore degree of freedom is two.
| F = C – P + 2 F = 1 – 1 + 2 F = 2 Thus the areas are bivariant. The area ABC represents solid CO2 The area BCD represents liquid CO2 The area ABD represents gaseous CO2 |
(3) Triple Point B- Point B is the triple point of CO2, at which all three phases of CO2 coexist and are in equilibrium with each other. The temperature at this point is -57°C and the pressure is 5.2 atm. At this point number of phases P = 3 and C = 1. Therefore degree of freedom is 0 as shown below:
| F = C – P + 2 F = 1 – 3 + 2 F = 0 |
Thus at this point, the system is invariant.
Difference Between Water and CO2 System:
(1) In the CO2 system, the fusion curve inclines away from the pressure axis whereas in the water system, it inclines towards the pressure axis (i.e., negative slope in water and positive slope in CO2).
(2) The vapor pressure of solid CO2 even at extremely low temperatures is very high and many times higher than ice.
(3) The pressure and temperature corresponding to the CO2 system are much higher than for the H2O system.







Comments (No)