Relative Density and Specific Gravity:
The heaviness or weight per unit volume of a substance is expressed in terms of its density. Since the weight of a substance depends on its mass, the term density may be defined as the mass of a substance per unit volume. Thus,
Density = Mass / Volume or D = M / V
Where ‘D’, ‘M’ and ‘V’ represent density, mass and volume respectively.
The density of one substance is different from that of another. Due to this reason, it is found convenient to mention the density of a substance in comparison with that of a reference substance selected as a standard. This ratio is referred to as relative density. The relative density of a substance may be defined as the ratio of its density to the density of a reference substance. Thus,
Relative Density (R.D.) = Density of a substance / Density of a reference substance
Being a ratio, the relative density has no unit. On the other hand, density is expressed in terms of kg/m3 (S.I. unit). When the reference substance is water in case of solids or liquids and hydrogen in case of gases, the relative density is referred to as specific gravity. The specific gravity may, thus, be defined as the ratio of a density of a substance to the density of water (for solids and liquids) or to the density of hydrogen (for gases).
Determination of Specific Gravity (or Relative Density) of Solids and Liquids:
(1) The specific gravity (or the relative density) of the solids and liquids can be determined with the help of Archimedes principle as given under-The specific gravity of a solid heavier than water. The given solid, say a glass stopper, is suspended from the hook of the shorter pan of a hydrostatic balance by means of a fine silk thread and weighed. This gives the weight of the solid in the air.
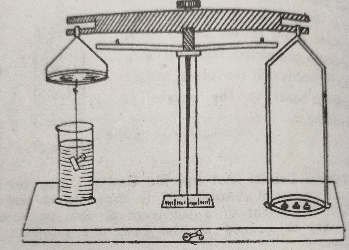
Now a beaker containing water is placed under the shorter pan in such a way that the solid dips in water completely. Now the solid is weighed while keeping it in water. This gives the weight of solid in water. Finally the specific gravity of the solid is calculated as under-
Weight of the solid in air = w1g
Weight of the solid in water = w2g
Loss of weight of solid in water = (w1-w2)g
Specific gravity of the solid = Weight of solid in air / Loss of weight of solid in water = w1 / w1-w2
(2) Specific gravity of a solid lighter than water. The given solid, say a cork, is first weighed alone in the air. Then it is tied to a sinker (some heavier body, say a glass stopper) and the weight of the given solid cork in air and sinker in water is determined. Finally both the given solid and the sinker are weighed together in the water. The loss in weight of the given solid is then determined and the specific gravity is calculated as under-
Weight of the given solid (say a cork) in air = w1g
Weight of the given solid in air and sinker in water = w2g
Weight of the given solid and sinker both in water = w3g
Loss of weight of given solid in water = (w2 – w3)g
Specific gravity of the given solid = Weight of given solid in air / Loss of weight of given solid in water = w1 / w2 – w3
(3) Specific gravity of liquid. Some solid, heavier than water, say a glass stopper, is at first weighed in air, then in water and finally in the given liquid. The loss of weight of solid in both the case is determined and the specific gravity is then calculated as under-
Weight of glass stopper in air = w1g
Weight of glass stopper in water= w2g
Weight of glass stopper in the given liquid = w3g
Loss of weight in water = (w1 – w2)g
Loss of weight in the given liquid = (w1 – w3)g
Specific gravity of given liquid = Loss of weight of glass stopper in liquid / Loss of weight of glass stopper in water = w1 – w3 / w1 – w2









Comments (No)