What is Structural Isomerism?
When two or more compound having the same molecular formula possesses different chemical structures i.e., different arrangement of atoms or group of atoms within the molecule, the phenomenon is known as structural isomerism and such compounds are called structural isomers.
Types of Structural Isomerism:
(1) Chain Isomerism- It is also known as nuclear isomerism or skeletal isomerism. It is shown by hydrocarbons. When two or more compounds having the same molecular formula but possess different arrangement of carbon atoms in the main chain are referred to as chain isomers and the phenomenon is known as chain isomerism.
Example- (a) Butane has two structural chain isomers having the molecular formula C4H10.

(b) Pentane exists in three chain isomers having the molecular formula C5H12.
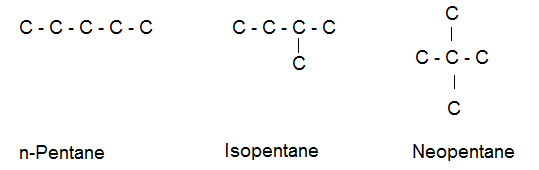
(2) Position Isomerism- Two or more compounds having the same molecular formula and structure of chain but have different positions of various functional groups or multiple bonds present in them are referred to as position isomers and the phenomenon exhibited by them is called positional isomerism.
Example- (a) 1-Chloropropane and 2-Chloropropane are position isomers.

(b) 1-Butene and 2-Butene.

(3) Functional Isomerism- Two or more compounds having the same molecular formula but differ in the functional groups in them are referred to as functional isomers and the phenomenon exhibited by them is called functional isomerism.
Various classes of organic compounds which show functional isomerism are as-
(a) Compounds having functional groups alcohols [-OH] and ethers [R-O-R]. Example- Butanol and Diethyl Ether having molecular formula C4H10O.

(b) Cyanide(-C≡N) and Isocyanide (-N≡C). Example- Propanenitrile and Ethyl Isocyanide.

(c) Nitroalkanes (R-NO2) and Alkyl Nitrites (RONO). Example- Nitropropane and Propyl Nitrite.

(d) Carboxylic acid (RCOOH) and esters (RCOOR). Example- Propanoic Acid and Methylethanoate.
(e) Dienes and alkynes. Example- Butadiene and 1-butyne.
| CH2 = CH – CH = CH2 ————–> CH3 – CH2 – C ≡ CH |
(4) Metamerism- Two or more compounds having the same molecular formula and same functional group but have a different number of carbon atoms or alkyl groups on either side of the functional group are referred to as metamers and the phenomenon exhibited by them is known as metamerism.
Example- (a) Diethylamine is a metamer of Methyl-n-propylamine.
| C2H5 – NH – C2H5 (Diethylamine, C4H11N) ————–> CH3 – NH – C3H7 (Methylpropylamine, C4H11N) |
(b) Diethylether is a metamer of Methylpropylether.
| C2H5 – O – C2H5 ————–> CH3 – O – C3H7 |
(c) Diethylthioether is a metamer of Methylpropylthioether.
| C2H5 – S – C2H5 ————–> CH3 – S – C3H7 |
(5) Tautomerism- It is also known as kryptomerism, allelotropism, desmotropism or metotropy. It is a special type of functional isomerism in which isomers exist in dynamic equilibrium with each other. It may be defined as the phenomenon in which a single compound exists in two readily interconvertible forms which differ markedly in the relative position of at least one atomic nucleus, generally hydrogen.
Structural requirement for Tautomerism-
(a) Compounds should have electronegative atom bonded with multiple bond i.e. N and O.
(b) Compound should have at least one acidic hydrogen present at α-carbon of the molecule.
If these two conditions are fulfilled then the compound will show tautomerism.
Tautomers are always in an equilibrium state.
The number of sigma, pi and lone pair of electrons in both tautomers are always the same.
Tautomerism is a chemical phenomenon that takes place only in a liquid state.
Tautomerism is catalysed by acid as well as a base.
The most important type of tautomerism is keto-enol tautomerism. In this type of tautomerism, one tautomer contains a keto group while the other contains the enolic group.

In this case, the enolic form is less stable. It is stabilized by intermolecular hydrogen bonding.








Comments (No)