Table of Contents
Atomic Orbital and Bond Order:
Concept of Atomic Orbital:
The behaviour of microscopic particles like an electron is now explained on the basis of a new concept known as “Quantum Mechanics” or “Wave Mechanics” which takes into consideration “Uncertainty Principle” as well as “dual nature of matter“. Keeping in view Bohr’s concept, de-Broglie’s dual concept and the Uncertainty principle, Erwin Schrodinger (1926) derived a mathematical relation called “Schrodinger’s Wave Equation” which describes the behaviour of an electron wave in a three-dimensional space around the nucleus of an atom. This equation is represented as-
∂2ψ/∂x2 + ∂2ψ/∂y2 + ∂2ψ/∂z2 + 8π2m/h2 (E-V)ψ = 0, where x, y, z are space (cartesian) coordinates, m = mass of electron, h = Planck’s Constant, E = total energy of the electron, V = potential energy of the electron, ψ = amplitude of electron wave known as wave function, ∂2ψ/∂x2 = second derivative of ψ with respect to x and so on.
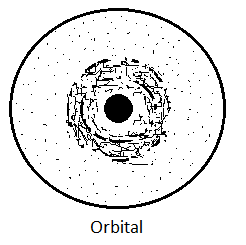
The solutions of this equation are known as Wave-functions (ψ) which have many values, but only certain values are significant and are known as Eigen functions. The wave function (ψ) itself has no significance, however, the square of this wave function (ψ2) gives the probability of finding the electron in different regions of space around the nucleus. The three-dimensional region of space around the nucleus in which the probability of finding the electron is maximum is known as “Orbital“. It is represented in terms of charge cloud whose density is proportional to ψ2 (probability of finding the electron). In this representation, an electron is considered as a very small dot and if a large number of photographs of the electron in an H-atom are taken at a very short time interval on the same film, then on developing the film, the picture obtained is as follows which is known as “Electron Cloud representation of Orbital“. It is clear from the figure that the probability of finding the electron is greater near the nucleus and decreases as the distance from the nucleus increases, but never becomes zero. However, for convenience, a boundary surface is drawn in which the probability is maximum (90-99%) known as Atomic Orbital.
Concept of Bond Order:
The concept of bond order was introduced to determine the relative stability of molecules which is defined as “half of the difference between the number of electrons in bonding molecular orbital and anti-bonding molecular orbital i.e.
Bond Order = nb – na/2
Where ‘nb’ is the number of electrons in bonding molecular orbital and ‘na’ is the number of electrons in an anti-bonding molecular orbital.
Significance of Bond Order:
The bond order convey the following important informations-
- A positive value of bond order indicates that the molecule is stable whereas a negative or zero bond order means that the molecule is unstable and is not formed.
- Greater the bond order, greater is the dissociation energy and vice versa. Thus, a molecule having a bond order of 2 has greater dissociation energy than a molecule with a bond order of one or one and a half.
- Bond length is inversely proportional to bond order of the molecule i.e. greater the bond order shorter will be the bond length.
- The bond order of a molecule is equal to the number of covalent bonds between the atoms in the molecules. It may have integral or non-integral values. The integral bond order of one, two or three corresponds to single, double or triple bonds respectively.
- A molecule containing unpaired electrons is Paramagnetic and in which all the electrons are paired is Diamagnetic. It has been found that if bond order is fractional, the molecule will be definitely Paramagnetic. However, if bond order is a whole number, the molecule may or may not be Paramagnetic.








Comments (No)