Table of Contents
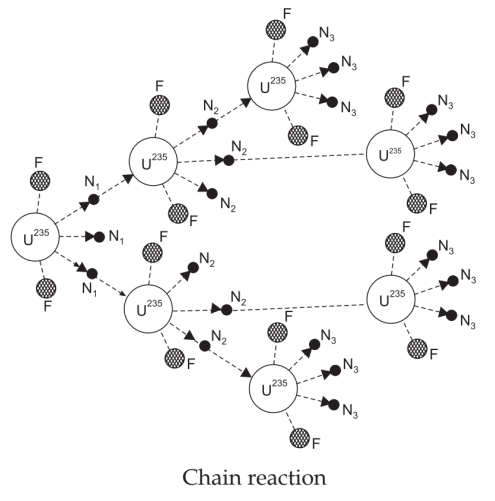
Chain Reaction in Nuclear Fission:
It is a self-maintaining process in which the nuclear fission of an atom induces nuclear fission in another atom, this further causes fission in the next more atoms and the process continues till whole the fissionable material is disintegrated.
Effective Multiplication Factor (k):
It is defined as the ratio of the rate of production of neutrons in the 2nd generation to the rate of loss (i.e. consumption) of neutrons in the 1st generation.
| i.e. k = P/(A + L) Where, A = rate of absorption of neutrons. L = rate of leakage of neutrons. |
If ‘F’ is the rate of fission reaction i.e. number of atoms disintegrating per second, then-
| P = NF Where ‘N’ is an average number of neutrons released/fission. ∴ k = NF/(A + L) |
- If k > 1, the chain reaction is self-sustaining but uncontrollable and there could be an explosion. Such assembly is called “super-critical“.
- If k = 1, the chain reaction is self-sustaining and controlled type. Such a system is called “critical“.
- If k < 1, the reaction stops at a certain time i.e. “sub-critical“.
Critical Mass- It is the size of fissionable material just necessary to maintain a self-maintained chain reaction.
Chain Reaction in Natural Uranium:
Natural uranium is a mixture of 92U235 (0.715%), 92U238 (99.28%) and 92U234 (0.006%).
The chain reaction in natural uranium is not possible.
Consider a mass of natural uranium which is so large that leakage of neutrons from its surface is practically nil. If a thermal neutron is introduced in this mass to cause fission, the neutrons released during the fission reaction are fast neutrons. These neutrons can suffer-
- Simple capture.
- Elastic or inelastic collision.
- or Can cause fission.
Repeated collisions of these fast neutrons with U238 nuclei will reduce their energy slowly. The energy lost in a single collision is very small because of the large mass difference between the U238 atom and neutron. This increases the probability of capture of neutrons by U238. On their capture, they would be lost forever and would not be available for inducing fission.
Thus chain reaction in natural uranium can be achieved by-
- Enriching uranium so that it contains more percentage of U235. This is used in the atom bomb.
- To cause to fission neutrons to slow down very rapidly. These neutrons can escape capture by U238 and cause fission in U235. This is used in a thermal reactor for producing nuclear power.
This is because the fission cross-section of U238 for slow or thermal neutrons is practically zero, while that of U235 is very high.









Comments (No)