Charles Law:
The volume of a given mass of a gas changes when its temperature is changed. Since the volume of a gas also changes with pressure, the volume-temperature relationship for a gas is studied at a constant pressure. During the experiment, the temperature is changed by dipping the syringe in a beaker containing water which can be cooled (by adding ice) or heated and the pressure is maintained constant by not changing the pressure applied on the piston, that is, without disturbing the piston externally. It has been observed that the volume of the enclosed gas or air increases on raising its temperature and decreases when its temperature is lowered.
Charles, a French scientist, was the first to discover the quantitative relationship between the volume of a given mass of a gas and temperature, at constant pressure. It was verified by Gay-Lussac. According to Charles, pressure remaining constant the volume of a given mass of gas expands or contracts by 1 / 273 of its volume at 0°C for every one-degree rise or fall in temperature.
A more precise value of the fraction is 1 / 273.16 but we shall take it as 1 / 273 for convenience sake.
If V0 is the volume of a gas at 0°C and Vt the volume at t°C, then we can write-
| Volume at t°C = Volume at 0°C + t / 273 x Volume at 0°C or Vt = V0 + t / 273 x V0 = V0 (1 + t / 273) Vt = V0 (273 + t / 273) ………….(a) |
According to this relation,
| 273 ml of any gas at 0°C becomes 274 ml at 1°C 275 ml at 2°C 373 ml at 100°C 272 ml at -1°C 173 ml at -100°C 2 ml at -271°C 1 ml at -272°C 0 ml at -273°C |
The volume of the gas is reduced to zero at -273°C, i.e., the gas disappears when this stage is reached. In an actual experiment, it has been observed that it never happens and before this condition is reached, the gas gets liquefied. This temperature (-273°C) on the Celsius scale, when the gas liquefied, is called the Absolute zero. A new temperature scale based on absolute zero as the starting point has been evolved and it is known as Absolute scale or Kelvin scale. This scale was given by Lord Kelvin. Absolute or Kelvin scale has one advantage that all the temperatures on this scale are in positive figures. The values on this scale are expressed in degrees absolute (°A) or degrees Kelvin (°K). However, the word degree or its sign (°) is usually not written while writing the absolute or kelvin temperatures. Thus, we can just write ‘A’ for absolute or ‘K’ for kelvin. Any temperature measured on Celsius scale can be converted into the temperature on the absolute or kelvin scale by adding 273 (more precisely 273.16) to it.
| Thus, 27°C + 273 = 300 A or K -73°C + 273 = 200 A or K Now, if V1 and V2 are the volumes of gas at temperature t1°C and t2°C, then according to the equation (a), we have V1 = V0 (273 + t1 / 273) ………..(b) V2 = V0 (273 + t2 / 273) ………….(c) Dividing (b) by (c), we get- V1 / V2 = V0 (273 + t1 / 273) / V0 (273 + t2 / 273) V1 / V2 = 273 + t1 / 273 + t2 = T1 / T2 Where T1 and T2 are the temperatures on the absolute or kelvin scale. or V1 / T1 = V2 / T2 Hence, V / T = a constant or V ∝ T (at constant pressure) |
This relationship between volume and temperature of a gas at constant pressure is known as Charle’s Law. It may be stated as under-
“Pressure remaining constant, the volume of a given mass of a gas is directly proportional to its absolute temeprature.”
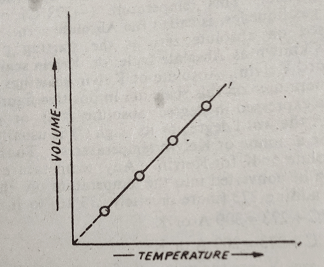
The variation of volume with the temperature at constant pressure can be illustrated graphically. A plot of the volume against temperature (in kelvin scale) gives a straight line which shows that V ∝ T at constant pressure. A constant pressure plot is called an isobar.









Comments (No)