Table of Contents
Preparation and Properties of Hydrogen Sulphide:
It is found in volcanic gases and in the waters of certain mineral springs. It is also formed as a result of the decomposition and decay of proteins present in living matter.
Preparation of Hydrogen Sulphide:
It is obtained by the methods as under-
- By action of hydrogen on sulfur.
| H2 + S ———–> H2S ↑ |
- By action of dilute HCl on zinc sulfide.
| ZnS + 2HCl ———–> H2S ↑ + ZnCl2 |
- By action of concentrated HCl on antimony sulfide.
| Sb2S3 + 6HCl ———–> 2SbCl3 + 3H2S ↑ |
- In the laboratory by the action of dilute HCl or H2SO4 on iron sulfide.
| FeS + 2HCl ———–> FeCl2 + H2S ↑ FeS + H2SO4 ———–> FeSO4 + H2S ↑ |
Method- A Woulfe’s bottle is fitted with a thistle funnel and a delivery tube as indicated in the figure-
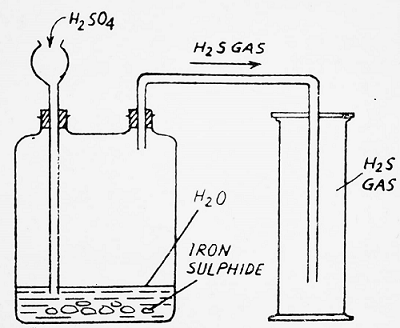
The pieces of iron sulfide are placed in the bottle and they are covered with water. Dilute H2SO4 is added through the thistle funnel. The reaction between FeS and H2SO4 takes place and hydrogen sulfide is obtained. It is collected in a gas jar by the upward displacement of air as it is heavier than air. It is not collected over water as it is soluble in water.
Properties of Hydrogen Sulphide:
Physical Properties of Hydrogen Sulphide:
- It is a colourless gas with a smell like that of rotten eggs.
- It is slightly heavier than air. Its density is 1.906.
- It is highly soluble in water.
- It is highly poisonous in nature. Small quantities produce headache. Large quamntities may cause even death.
- It can be liquefied to a colourless liquid (boiling point -60.7°C) and solidified to a transparent solid (melting point -85.50°C ).
Chemical Properties of Hydrogen Sulphide:
Chemically, H2S is a reactive compound.
(1) Combustion- It is a combustible gas and burns with a pale blue flame. It is not a supporter of combustion. In a limited supply of air or oxygen, it burns to give sulfur and water.
| 2H2S + O2 ———–> 2H2O + 2S |
In excess of air or oxygen, it burns to yield sulfur dioxide and water.
| 2H2S + 3O2 ———–> 2H2O + 2SO2 |
(2) Decomposition- On heating, it undergoes decomposition to give hydrogen and sulfur. Decomposition starts at 310°C and gets completed at 1700°C.
| H2S ———–> H2 + S |
(3) Acidic Properties- The gas is acidic in nature and turns moist blue litmus paper red. The aqueous solution of H2S is acidic in character due to H3O+ ions.
| H2S + H2O ———–> H3O+ + HS– HS– + H2O ———–> H3O+ + S– |
The acidic nature of the gas is supported by the following reactions.
(a) Action of alkalis- It reacts with caustic alkalis like NaOH to give two series of salts.
| H2S + NaOH ———–> NaHS (Sodium Hydrosulfide) + H2O H2S + 2NaOH ———–> Na2S (Sodium Sulfide) + 2H2O |
Being a weaker acid than carbonic acid (H2CO3), it cannot decomposes the carbonates.
(b) Action of ammonia- On treatment with NH3, ammonium sulfide is formed.
| 2NH3 + H2S ———–> (NH4)2S |
(c) Action of metals- It reacts with metals such as Ag or Sn to give the corresponding metal sulfide and hydrogen gas.
| 2Ag + H2S ———–> Ag2S + H2 ↑ Sn + H2S ———–> SnS + H2 ↑ |
(4) Reducing Properties- Hydrogen sulfide is a strong reducing agent owing to the ease with which it decomposes to give hydrogen and sulfur. Some important reducing reactions of hydrogen sulfide are as under-
(a) Action of halogens- Halogens are reduced to halogen acids.
| H2S + Cl2 ———–> 2HCl + S H2S + Br2 ———–> 2HBr + S |
(b) Action of sulfur dioxide- It reduces SO2 to sulfur.
| 2H2S + SO2 ———–> 2H2O + 3S |
(c) Action of ferric sulfate- Ferric sulfate is reduced to ferrous sulfate.
| Fe2(SO4)3 + H2S ———–> 2FeSO4 + H2SO4 + S |
(d) Action of H2SO4– It reduces H2SO4 to SO2.
| H2S + H2SO4 ———–> 2H2O + SO2 + S |
(e) Action of HNO3– In this case, NO2 is formed.
| H2S + 2HNO3 ———–> 2H2O + 2NO2 + S |
(f) Action of acidified KMnO4– It decolourises the pink colour of the KMnO4 solution.
| 2KMnO4 + 3H2SO4 + 5H2S ———–> K2SO4 + 2MnSO4 + 8H2O + 5S |
(g) Action of acidified K2Cr2O7– K2Cr2O7 solution is turned green.
| K2Cr2O7 + 4H2SO4 + 3H2S ———–> K2SO4 + Cr2(SO4)3 + 7H2O + 3S |
(5) Precipitation Reactions- It reacts with the solutions of metallic salts giving rise to the formation of coloured precipitates of insoluble sulfides. Herein, two cases arise-
(a) Precipitation reactions in acid medium- Hydrogen sulfide reacts with salts of the metals like Cu, Bi, Cd, As etc. in presence of dilute HCl to give a coloured precipitate of their sulfides.
| CuSO4 + H2S ———–> CuS (Black precipitate) + H2SO4 CdSO4 + H2S ———–> CdS (Yellow precipitate) + H2SO4 HgCl2 + H2S ———–> HgS (Black precipitate) + 2HCl Pb(NO3)2 + H2S ———–> PbS (Black precipitate) + 2HNO3 |
(b) Precipitation reactions in alkaline medium- Hydrogen sulfide reacts with salts of metals like Co, Ni, Zn etc. in ammoniacal medium (NH4OH) to give coloured precipitates of their sulfides.
| ZnCl2 + H2S ———–> ZnS (White precipitate) + 2HCl NiSO4 + H2S ———–> NiS (Black precipitate) + H2SO4 |
Uses of Hydrogen Sulfide:
Hydrogen sulfide is used-
- As an important analytical reagent in qualitative inorganic analysis.
- As an important reducing agent.
- In the presence of sulfides of metals like copper, antimony, tin and cadmium.
- In the manufacture of sulfuric acid for removing arsenic as arsenic sulfide. Oxygen and sulfur form hydrides namely hydrogen oxide or water and hydrogen sulfide, respectively. These hydrides are quite different in their properties.








Comments (No)