Table of Contents
Manufacture of Sulfuric Acid (H2SO4):
Sulfuric acid is the most important of all the acids. It plays a vital role in the industrial development and hence the economy of a country. As a matter of fact, it is referred to as the king of all chemicals. Sulfuric Acid is present in certain mineral springs and is produced as a result of the interaction of water on sulfides like iron pyrites.
2FeS2 + 2H2O + 7O2 ——–> 2FeSO4 + 2H2SO4
Sulfuric acid is obtained on a commercial scale by the following two methods- lead chamber process and contact process.
Lead Chamber Process:
This is an old process of manufacturing sulfuric acid.
Theory of lead chamber process– The theory underlying lead chamber process is as under-
(a) Production of sulfur dioxide- It is produced by burning sulfur powder or iron pyrites in excess of air.
S + O2 ——> SO2
4FeS2 + 11O2 ———> 2Fe2O3 + 8SO2
(b) Oxidation of sulfur dioxide to sulfur trioxide- SO2 is oxidized to SO3 by nitrogen dioxide. Nitric oxide so formed combines with oxygen of the air to give nitrogen dioxide.
SO2 + NO2 ——-> SO3 + NO
2NO + O2 ——–> 2NO2
(c) Conversion of sulfur trioxide into sulfuric acid- Sulfur trioxide is dissolved in water to yield sulfuric acid.
SO3 + H2O ——-> H2SO4
The plant used for the manufacture of sulfuric acid by lead chamber process is indicated in the figure-
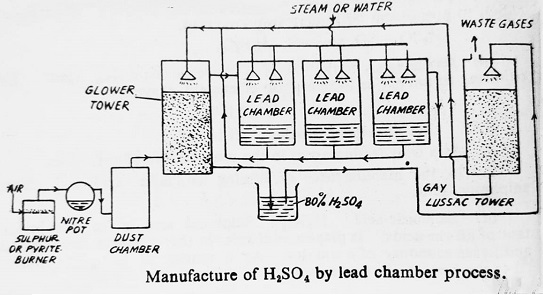
In this process, a mixture of SO2, air, steam and oxides of nitrogen is passed through a series of lead chambers. SO2 is oxidized to SO3 by oxides of nitrogen which function as a catalyst. Sulfur trioxide so formed then reacts with steam (H2O) to form sulfuric acid. This acid is referred to as chamber acid and its strength is about 60-70%.
Contact Process:
This is the modern process for the manufacture of sulfuric acid. It is largely used in the production of sulfuric acid these days.
Theory of contact process: The theory underlying the contact process is as under-
(a) Production of sulfur dioxide- Sulfur dioxide is obtained by burning of sulfur powder or iron pyrites in excess of air.
S + O2 ——> SO2
4FeS2 + 11O2 ———> 2Fe2O3 + 8SO2
(b) Conversion of sulfur dioxide into sulfur trioxide- SO2 is oxidized to SO3 by oxygen of air in the presence of platinized asbestos or vanadium pentoxide at 450°C.
2SO2 + O2 ⇌ 2SO3 + 45 kcals.
The above-cited reaction is reversible, exothermic and is accompanied by a decrease in volume. According to Le-Chatelier’s principle, the conditions for maximum yield of sulfur trioxide are as under-
- Low temperature- Low and optimum temperature (450-500°C) is maintained to get maximum yield of SO3.
- High Pressure- A pressure of 2 atmospheres is maintained during the process.
- Use of a catalyst- Platinized asbestos or V2O5 is used as a catalyst to increase the rate of reaction.
- Excess of oxygen- Excess of oxygen should be used during the process.
- Purity of gases- The gases needed like SO2 and O2 should be pure and dry.
(c) Conversion of sulfur trioxide into pyrosulfuric acid- SO3 is absorbed in concentrated H2SO4.
SO3 + H2SO4 ———> H2S2O7 (Pyrosulfuric acid or oleum)
(d) Conversion of H2S2O7 into H2SO4– Sulfuric acid of the desired concentration is obtained by diluting oleum with water.
H2S2O7 + H2O ——-> 2H2SO4
The plant used for the manufacture of sulfuric acid by the contact process is shown in the figure-
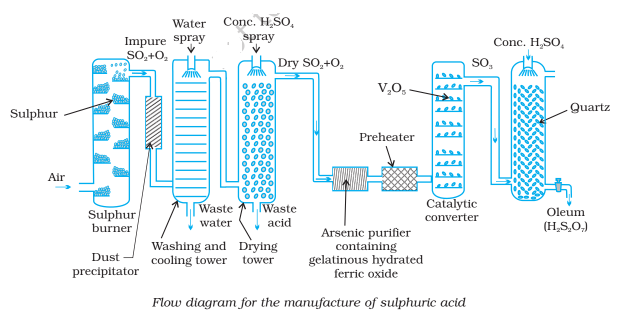
In this method, a dry and pure mixture of sulfur dioxide and the air is passed over heated platinized asbestos or vanadium pentoxide as a catalyst in the contact chamber. Sulfur dioxide is converted into sulfur trioxide by the oxygen of the air. Sulfur trioxide so formed is dissolved in concentrated H2SO4 to obtain pyro-sulfuric acid (oleum). It is then diluted with water to get sulfuric acid of any desired concentration.
Uses of Sulfuric Acid:
It is used-
- In the manufacture of fertilizers like (NH4)2SO4 and calcium superphosphate.
- In the manufacture of rayon, paints and pigments.
- In the refining of petroleum products.
- As a dehydrating agent in the laboratory and other research institutes.
- In the extraction of metals like copper and zinc.
- In the manufacture of the acids like HCl, H3PO4, HNO3.
- As an electrolyte in storage batteries and electroplating bath.
- In the manufacture of explosives like TNT, picric acid and dynamite.
- In the tanning of leather in industry.
- As a pickling reagent in enamelling and galvanizing process.
- In the production of a large number of organic compounds.
- In the medicines in the dilute state.








Comments (No)