What is Covalent Bond?
In the case of many compounds, the atoms attain a stable electronic configuration of the nearest inert gas by the mutual sharing of electrons between the combining atoms, each atom providing an equal number of electrons for the resulting covalent bond formation. The shared electrons contribute towards the stability of both the atoms involved in the chemical combination. A covalent bond is, thus, formed by the mutual sharing of an equal number of electrons between the atoms of the same or different non-metallic elements. The phenomenon of the formation of covalent compounds through covalent bonding is referred to as Covalency. The number of electrons shared by an atom to form a covalent compound determines its covalency.
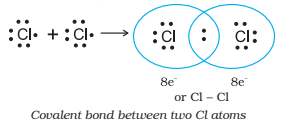
As a typical instance of covalent bond is the formation of a chlorine molecule from two chlorine atoms. Chlorine atom contains seven electrons in the outermost shell. In formation of a chlorine molecule, each chlorine atom contributes one electron and the resultant pair of electrons is then shared by both the chlorine atom, each atom attains the Ar configuration.
Characteristics of Covalent Compounds:
The covalent compounds show the important characteristics as under-
- Nature- The covalent compounds are mostly gases or liquids under ordinary conditions. They are made up of electrically neutral molecules. The forces of attraction between covalent molecules are much weaker than the forces of electrostatic attraction involved in electrovalent compounds.
- Low melting and boiling points- The covalent compounds possess low melting and boiling points. This is due to the fact that the forces of attraction involved in covalent compounds are much weaker than the forces of electrostatic attraction in electrovalent compounds.
- Solubility- The covalent compounds are usually sparingly soluble or insoluble in water but are soluble in organic solvents (non-polar solvents) such as benzene, ether, alcohol and chloroform.
- Non-ionic nature- The covalent compounds do not form ions when dissolved in solvents. They are made up of electrically neutral molecules and no charged ions are present in covalent compounds.
- Electrical Conductivity- In solution or in a molten state, the covalent compounds are bad conductors of electricity because the covalent compounds are made up of electrically neutral molecules and they contain no ions to act as carriers of electric current.
- Space Isomerism- In covalent compounds, the covalent bond is rigid and directional in nature. Hence, these compounds are capable of exhibiting the phenomenon of space isomerism.
- Stability- The covalent compounds are much less stable to heat in comparison to the electrovalent compounds.
- Coordinate covalent bond- In many compounds, a bond may be formed by sharing of two electrons which are contributed by only one of the bonding atoms. The atom which donates a pair of electrons is referred to as donor while the other atom which accepts the pair of electrons is called the acceptor. The coordinate covalent bond is represented by an arrow with its head pointing towards the acceptor atom. In general,
A : + B ———> A : B or A ———–> B
Herein, the atom A has a lone pair of electrons and is called a donor, while the atom B, which accepts the lone pair of electrons, is known as acceptor. Sometimes, one or more lone pairs of electrons are available with one of the participating atoms.
- Group 16 Elements (Oxygen Family)
- Group 15 Elements (Nitrogen Family)
- Group 14 Elements (Carbon Family)
- Group 13 Elements (Boron Family)
- Group 2 Elements (Alkaline Earth Metals)
- Group 1 Elements (Alkali Metals)
- Group 17 Elements (Halogen Family)
- Group 18 Elements (Noble Gases)
- Modern ABC of Chemistry Class-12 Part I & Part II (Set of 2 Books)









Comments (No)