Energy Bands in Solids:
The electrons of an isolated atom have discrete energy levels. When a large number of atoms are brought closer, then the valence electrons of all these atoms interact with each other. Due to this, the discrete energy levels of all the valence electrons of all these atoms come very close to each other. These closely spaced energy levels form the Energy Bands.
Consider a Silicon Crystal with N-atoms.
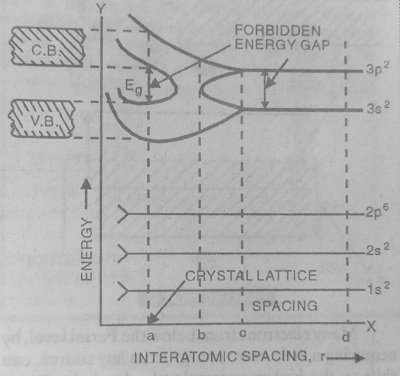
The electronic configuration of Si (z = 14) is 1s2 2s2 2p6 3s2 3p2. In this case, the 1st and 2nd orbits are completely filled with electrons. Here 3S subshells is completely filled with 2 electrons whereas 3p subshell contains only 2 electrons. However, it can accommodate 6 electrons.
In Silicon Crystal, there are 14N electrons (or 14N Energy Levels). In this case, the 1st and 2nd orbits are completely filled with energy levels. Here 3s subshell is completely filled with 2N energy levels whereas the 3p subshell contains only 2N Energy levels. However, it can accommodate 6N energy levels.
Let us discuss the different situations-
- If the interatomic distance of the atom is very large (i.e. r = d), then there is no interatomic interaction.
- When the interatomic distance is equal to ‘c’, the interactions between the valence electrons of various Silicon atoms becomes appreciable.
- When the interatomic distance is equal to ‘b’, the energy gap between 3s and 3p levels completely disappears. Now the energy levels of 3s subshell and 3p subshell mix with each other. In such a situation, it is not possible to distinguish between the electrons belonging to 3s and 3p subshell. We can only say that there are 8N energy levels in which 4N energy levels are filled and 4N energy levels are empty.
- When the interatomic distance ‘r’ is equal to ‘a’, (the actual interatomic distance in the crystal), then the energy band containing 4N filled energy levels is separated from the energy band which contains 4N empty energy levels. The energy gap between the filled energy band and the empty energy band is called the forbidden gap. The lower completely filled band is called the valence band and the upper unfilled band is called the conduction band.








Comments (No)