Table of Contents
Importance of Macromolecules:
A number of macromolecules such as carbohydrates, lipids (fats), proteins (structural proteins and enzymes), and nucleic acids occur in living systems. Their molecular weight varies from several hundred to several million units. Macromolecules are formed by polymerization of smaller units.
Carbohydrates: The Main Energy Storage Molecules
Carbohydrates are compounds of carbon, hydrogen, and oxygen. They are mainly of three types: monosaccharides, oligosaccharides, and polysaccharides.
(1) Monosaccharides- These are the simplest carbohydrates that cannot be further hydrolyzed into smaller units. Examples are Glucose, fructose, ribulose, mannose, erythrose, etc.
(2) Oligosaccharides- These are formed by the joining together of 2-6 molecules of monosaccharides. These can be hydrolyzed by an acid or enzyme. Examples are sucrose, lactose, and maltose.
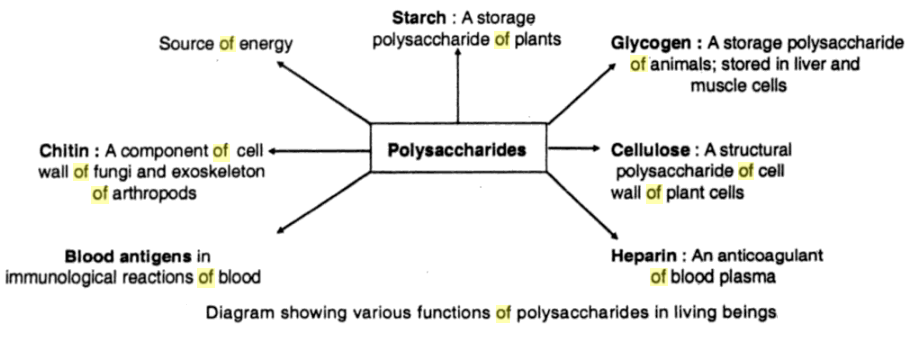
(3) Polysaccharides- These are formed by joining together more than six molecules of monosaccharides. Examples are glycogen, starch, and cellulose. Cellulose is the most abundant organic compound found in nature and is the structural carbohydrate of plants.
Fats and Lipids: Major Group of Insoluble Hydrocarbons having Many Functions
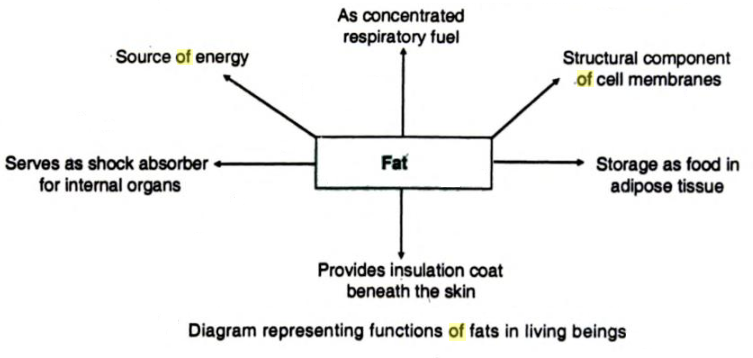
These are also compounds of carbon, hydrogen, and oxygen, but have much less oxygen as compared to carbohydrates. The fats are oily in nature. Some are solid and the others are liquid at room temperature. They serve as fuel and form structural components of the cell membranes. Fat is deposited just under the skin and acts as an insulating layer.
Cooking oil, butter, ghee, waxes, cholesterol, essential oils and natural rubber are examples of lipids. Lipids form 3-5% of the cell contents.
Nucleic Acids: Information Storage Devices of Cells
Nucleic acids are giant macromolecules with complex structure. These are compounds of carbon, hydrogen, oxygen and nitrogen. These are polymers of nucleotides and are larger than most proteins. They have a very high molecular weight. There are four kinds of nucleotides. Each nucleotide contains a nitrogenous base connected to a pentose sugar and a molecule of phosphoric acid.
Nucleic acids are of two kinds: Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). DNA is the genetic material in all the organisms. RNA is the genetic material in plant viruses and a few animal viruses.
Proteins: Structural and Functional Makeup of Cells
These are compounds of carbon, hydrogen, oxygen, nitrogen, and usually sulfur and phosphorous. These are most diverse in form and function. These are of prime importance because:
(1) these form the structural framework of protoplasm.
(2) are responsible for the colloidal nature of protoplasm.
(3) act as biocatalysts or enzymes and control almost all the biochemical reactions occurring in the body.
(4) form certain hormones that regulate the growth and functioning of various body activities.
Proteins are polymers of amino acids. The number of amino acid molecules in a protein molecule may vary from a few to several thousand. In all, twenty different amino acids form regular constituents of infinite varieties of proteins occurring in living beings.








Comments (No)