Table of Contents
Nature of Radiations from Radioactive Substances:
The nature of rays emitted by radioactive substances was determined by Ernest Rutherford (1904). He placed a sample of uranium (radioactive element) mineral in a lead box which absorbs all other radiations except those moving directly upwards. These radiations emitted by the uranium mineral were subjected to the action of the strong electric field when they split up into three types-
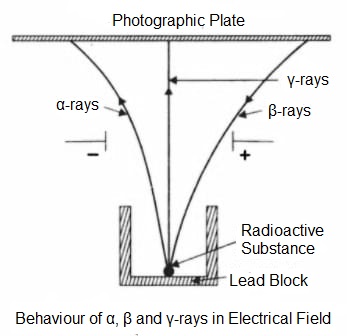
- The rays which are slightly deflected towards the negative plate were named alpha (α) rays which are composed of positively charged α-particles.
- The rays which are heavily deflected towards the positive plate were named Beta (β) rays which are composed of negatively charged β-particles.
- The rays which remained undeflected were named Gamma (γ) rays.
Thus, radioactive radiations given off by a radioactive substance are of three types i.e. α-rays, β-rays and γ-rays.
Characteristics of Alpha, Beta and Gamma-rays:
Alpha-rays:
- Nature- These are positively charged particles carrying 2 units of positive charge and 4 units of mass (i.e. contain 2 Protons and 2 Neutrons. So, it is simply He-nuclei 2He4).
- Effect of External Electric Field-These are slightly deflected towards the negatively charged plate due to their high momentum.
- Velocity-These travel with a velocity of about 1/10th of the velocity of light.
- Penetrating Power- These have less penetrating power due to their large mass. These can be easily stopped by a thin sheet of paper or Al-foil about 0.1 mm thick.
- Kinetic Energy- These have very high kinetic energy due to their heavy mass and high velocity.
- Ionising Power- These have quite high ionising power because due to their high kinetic energy, they cause intense ionisation of the gas through which they pass causing knocking out of the electrons from the molecules of the gases.
- Effect on Photographic Plate- Due to their high kinetic energy, α-particles affect the photographic plate to a large extent.
- Effect on Zinc Sulfide Plate- These produce luminosity on the ZnS plate due to their high kinetic energy (i.e. flashes of light are produced).
Beta-rays:
- Nature- These consist of negatively charged particles carrying a unit negative charge and negligible mass. Their e/m (charge/mass) ratio is the same as that for electrons, therefore, β-particles are simply electrons -1e0 or -1β0.
- Effect of External Electric Field- These are heavily deflected towards the positively charged plate due to their low momentum.
- Velocity- These travel with a velocity almost equal to that of light.
- Penetrating Power- These are 100 times more penetrating than α-rays because of their negligible mass and high velocity. These can pass through a 0.2 cm thick Al-sheet or mica sheet about 5 mm thick.
- Kinetic Energy- Thes have low kinetic energy than α-rays; though their velocity is very high but the mass is very small.
- Ionising Power- These have ionising power about 1/100th of that of α-particles due to their low kinetic energy.
- Effect on Photographic Plate- β-particles affect the photographic plate more than α-particles, though their kinetic energy is low. This is due to the fact that β-rays on being stopped by material object changes into x-rays having very strong photographic activity.
- Effect on Zinc Sulfide Plate- These have very little effect on the ZnS plate due to their low kinetic energy.
Gamma-rays:
- Nature- These rays do not consist of material particles and are electrically neutral. In fact, these are electromagnetic waves of very short wavelength (10-8 – 10-11 cm).
- Effect of External Electric Field- These remain undeflected.
- Velocity- These travel with a velocity equal to that of light (3.0 X 108 m/sec).
- Penetrating Power- These are the most penetrating of all the radioactive radiations because of their high velocity and small wavelength. These can easily penetrate through a 25 cm thick Fe sheet or 100 cm thick Al-sheet.
- Kinetic Energy- Due to their non-material nature, their kinetic energy is quite low.
- Ionising Power- These have very poor ionising power due to their non-material nature.
- Effect on Photographic Plate- These have very little effect on the photographic plate due to their very low kinetic energy.
- Effect on Zinc Sulfide Plate- These have very little effect on the ZnS plate due to their very low kinetic energy.









Comments (No)