Table of Contents
Preparation and Properties of Oxygen:
Oxygen is a colourless gas. It occurs in the free state in the air. About 1/5 of the air is oxygen by volume. In the combined state, it is present in water, the earth’s crust, plants and animal tissues.
Preparation of Oxygen:
Oxygen is prepared by a number of methods as under-
- By heating oxides of mercury and silver. Example-
| 2HgO ———-> 2Hg + O2 2Ag2O ———-> 4Ag + O2 |
- By heating compounds rich in oxygen. Example-
| 2KNO3 ———-> 2KNO2 + O2 2KMnO4 ———-> K2MnO4 + MnO2 + O2 |
- By action of water on peroxides of metals. Example-
| 2Na2O2 + 2H2O ———-> 4NaOH + O2 |
- By action of concentrated H2SO4 on MnO2, KMnO4, etc.
| 2MnO2 + 2H2SO4 ———-> 2MnSO4 + 2H2O + O2 |
- In the laboratory, oxygen is obtained by heating a mixture of potassium chlorate and manganese dioxide.
| 2KClO3 + MnO2 ———-> 2KCl + MnO2 + 3O2 |
Herein, MnO2 acts as a catalyst. A catalyst is a substance that does not undergo any chemical change in course of a chemical reaction.
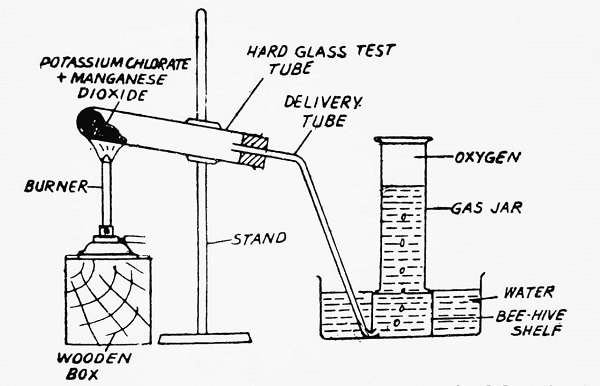
Method- Take a hard glass test tube and fill it with a mixture of KClO3 and MnO2 in the ratio of 4:1 by weight. Fit up the apparatus as indicated in the figure. Heat the test tube at first gently and then strongly. The evolution of oxygen gas takes place. It is collected in a gas jar by the downward displacement of water.
Properties of Oxygen:
Physical Properties of Oxygen:
- Oxygen is a colourless, odourless and tasteless gas.
- It is slightly soluble in water. The solubility of oxygen in a litre of water is 32.2ml at 15°C and 760 mm pressure. The aquatic animals breathe oxygen dissolved in water into their blood streams and thus, can live under water.
- It is slightly heavier than air. Its density at 0°C and 760 mm pressure is 1.429 g/litre.
- It can be liquefied to a pale blue liquid (boiling point -183°C) and solidified to a blue solid (melting point -218°C).
- It dissolves in alkaline pyrogallol solution.
Chemical Properties of Oxygen:
Chemically, oxygen is a reactive element.
(1) Nature- Oxygen neither turns blue litmus paper red nor red litmus paper blue but is neutral to litmus paper.
(2) Combustion- Oxygen is non-combustible but is a supporter of combustion. It does not burn itself but helps in burning other substances in its presence.
(3) Action of non-metals- Oxygen combines with non-metals like C, S, P etc. to give rise to the formation of the corresponding acidic oxides.
| C + O2 ———-> CO2 S + O2 ———-> SO2 P4 + 5O2 ———-> 2P2O5 2H2 + O2 ———-> 2H2O |
These acidic oxides when dissolved in water yield the corresponding acids.
| CO2 + H2O ———-> H2CO3 (Carbonic acid) SO2 + H2O ———-> H2SO3 (Sulfurous acid) P2O5 + 3H2O ———-> 2H3PO4 (Phosphoric acid) |
(4) Action of metals- It combines with metals like Na, Mg, Fe, etc. to form the corresponding basic oxides.
| 4Na + O2 ———-> 2Na2O (Sodium oxide) 2Na + O2 ———-> Na2O2 (Sodium peroxide) 2Mg + O2 ———-> 2MgO 4Fe + 3O2 —Ordinary Temperature——-> 2Fe2O3 3Fe + 2O2 —High Temperature——-> Fe3O4 |
These basic oxides when dissolved in water give rise to the formation of corresponding hydroxides.
| Na2O + H2O ———-> 2NaOH 2Na2O2 + 2H2O ———-> 4NaOH + O2 MgO + H2O ———-> Mg(OH)2 |
(5) Action of compounds- Certain compounds combine with oxygen in the presence of a certain catalyst to give the corresponding products.
(a) Action of SO2– SO2 is oxidized to SO3 at 450°C in presence of a catalyst like V2O5 or platinized asbestos.
| 2SO2 + O2 ⇌ 2SO3 |
(b) Action of NH3– Ammonia is oxidized to nitric oxide at 800°C in the presence of a catalyst like platinum gauze.
| 4NH3 + 5O2 ⇌ 4NO + 6H2O |
(c) Action of HCl- HCl gas is oxidized to chlorine in the presence of a catalyst like cupric chloride.
| 4HCl + O2 ⇌ 2Cl2 + 2H2O |
(6) Formation of Ozone- On passing a silent electric discharge through pure and dry oxygen at a lower temperature, ozone is formed.
| 3O2 ⇌ 2O3 (Ozone) |
Like phosphorous and sulfur, oxygen also shows the phenomenon of allotropy. Ozone is an allotropic form of oxygen. The upper layers of the atmosphere contain a layer of ozone that absorbs cosmic radiations. These radiations are harmful to living beings on the earth. Thus, living beings are protected from these harmful radiations. Ozone is highly unstable and decomposes to give oxygen.
| 2O3 ———-> 3O2 |
Uses of Oxygen:
- In hospitals for artifical respirations.
- As a liquid in rocket.
- In the production of oxy-hydrogen or oxy-acetylene flames for welding and cutting.
- In breathing apparatus for miners, deep-sea diviers, aviators and mountaineers.
- In iron and steeel industry.
- As an oxidizing agent in the laboratory.
- As an anaeshtetic in combination with nitrous oxide or ethylene.









Comments (No)