Table of Contents
Sulfur Dioxide Preparation and Physical Properties:
Sulfur dioxide is present in volcanic gases and in gases from certain mineral springs. It is also found in traces in the air of many cities and industrial towns and is formed due to the burning of the sulfur present as an impurity in coal.
Preparation of Sulfur Dioxide:
Sulfur dioxide is prepared by the methods described as under-
(a) By burning of sulfur in air or oxygen. Example-
| S + O2 ————-> SO2 ↑ |
(b) By heating a metallic sulfide in an excess of air.
| 2ZNs + 3O2 ————-> 2ZnO + 2SO2 2PbS + 3O2 ————-> 2PbO + 2SO2 4FeS2 + 11O2 ————-> 2Fe2O3 + 8SO2 |
(c) By action of dilute HCl or H2SO4 on metal sulfites or bisulfites.
| Na2SO3 + 2HCl ————-> 2NaCl + H2O + SO2 2NaHSO3 + H2SO4 ————-> Na2SO4 + 2H2O + 2SO2 |
(d) By reduction of concentrated H2SO4 on heating with carbon, sulfur or mercury.
| C + 2H2SO4 ————-> CO2 +2H2O + 2SO2 S + 2H2SO4 ————-> 2H2O + 3SO2 |
(e) In the laboratory by heating copper turnings with concentrated H2SO4. The reaction involved is-
| Cu + 2H2SO4 ————-> CuSO4 + 2H2O + SO2 |
Method- A round-bottomed flask is fitted with a cork having a double bore through one of which passes a thistle funnel and through the other a delivery tube, bent twice at right angles. This is indicated in the figure-
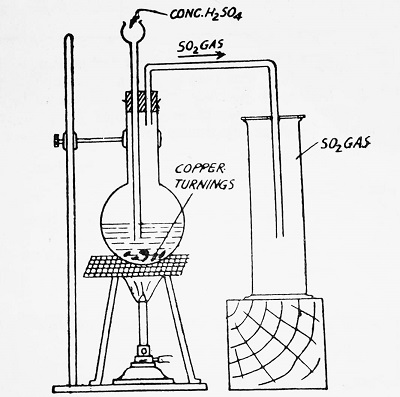
Copper turnings are taken in the flask and concentrated H2SO4 is added through the thistle funnel so that the lower end of the funnel is dipped in the acid. On heating the flask, the reaction between copper turnings and H2SO4 takes place. Sulfur dioxide is evolved, which is collected by the upward displacement of air as the gas is heavier than air. It is not collected over water as it is exceedingly soluble in water.
Physical Properties of Sulfur Dioxide:
- It is a colourless gas having a characteristic pungent suffocating smell like that of burning sulfur.
- It is about 2.26 times heavier than air.
- It is posionous in nature and causes inflammation of lungs and asthama.
- It is exceedingly soluble in water. 1 volume of water can dissolve 40 volumes of SO2 at 20°C. The aqueous solution of the gas is acidic in nature and has a sour taste. This is referred to as sulfurous acid (H2SO3).
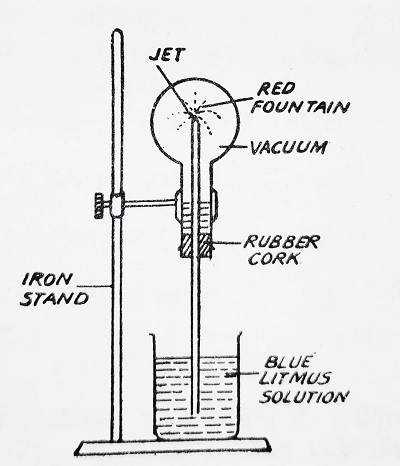
The acidic nature of the gas is demonstrated by the Fountain experiment.
Fountain Experiment- Fill a round-bottomed flask with sulfur dioxide gas. Insert through a cork a long glass tube having a jet. Support the flask upside down using an iron stand and closing the open end of the tube. Now remove the finger under blue coloured water (made by adding blue litmus solution) in a beaker. When it rises into the flask, in the form of a fountain, the solution acquires a red colour indicating the acidic nature of the gas and also indicating that SO2 gas is extremely soluble in water.
- It can be liquefied to a colourless liquid (boiling point -10°C) and solidified to a snow white solid (melting point -76°C).








Comments (No)