Table of Contents
Crystal Lattice and Unit Cell:
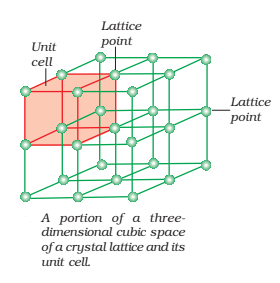
Crystal Lattice or Space Lattice:
A crystal can be represented by a regular three-dimensional arrangement of points which represent the constituent particles in space. Such a regular array of points in three dimensions is called Crystal Lattice or Space Lattice. It is usually represented by lines and dots which represent the position of constituent particles and known as Lattice points. Each lattice point has the same environment as that of any point in the lattice.
Unit Cell:
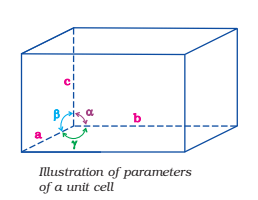
It is possible to select a group of lattice points in a crystal lattice which when repeated again and again, give the complete structure of solid. Such a part of space lattice is called a unit cell. Thus, a unit cell is defined as the three-dimensional group of lattice points which when repeated in space generates the crystal structure. Each unit cell is characterized in terms of distance ‘a‘, ‘b‘ and ‘c‘ along three edges of unit cell known as axial lengths and angle α, β and γ between the pairs of edges (b, c), (c, a) and (a, b) respectively known as axial angles.
Seven types of Unit Cells or Primitive Unit Cells are:
| System | Axial Distance | Axial Angles | Examples |
|---|---|---|---|
| Cubic | a = b= c | α = β = γ = 90° | NaCl, KCl, ZnS, Cu, Ag etc. |
| Tetragonal | a = b ≠ c | α = β = γ = 90° | SnO2, TiO2, NH4Cl etc. |
| Orthorhombic | a ≠ b ≠ c | α = β = γ = 90° | KNO3, K2SO4, BaSO4 etc. |
| Monoclinic | a ≠ b ≠ c | α = γ = 90°, β ≠ 90° | Na2SO4.10H2O, CuSO4.2H2O etc. |
| Triclinic | a ≠ b ≠ c | α ≠ β ≠ γ ≠ 90° | CuSO4.5H2O, H3PO3 etc. |
| Rhombohedral | a = b= c | α = β = γ ≠ 90° | NaNO3, Quartz, etc. |
| Hexagonal | a = b ≠ c | α = β = 90°, γ = 120° | Graphite, ZnO, CdS, etc. |
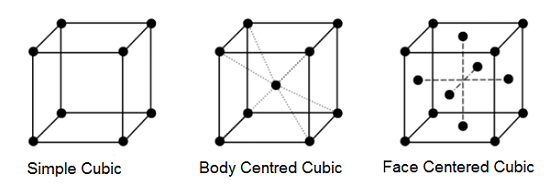
Cubic Crystal System:
There are three types of cubic crystal systems-
- Simple Unit Cell- A unit cell having lattice points only at the corner is called a simple or primitive or basic unit cell.
- Body Centred Unit Cell- A unit cell having lattice points at the centre of the cube in addition to the corner of the cube is called a body centred unit cell.
- Face Centered Unit Cell- A unit cell having lattice points at the centre of each face of the unit cell in addition to the corners of a cube is called face centered unit cell.
Calculation of Number of Particles in a Unit Cell:
- Each particle at the corner is shared by eight unit cells in the lattice and hence, it contributes 1/8th to a particular unit cell.
- A particle at the edge centre is shared by four unit cells in the lattice and hence, its contribution is 1/4th to a particular unit cell.
- A particle at the centre of the face of a unit cell is shared by two unit cells in the lattice and contributes only 1/2 to a particular unit cell.
- A particle at the body centre of a unit cell belongs only to that unit cell.
Example-
- In a simple cubic unit cell, there are 8 atoms at the corners of the cube and each atom makes 1/8 contribution to the unit cell. Thus, the number of atoms in this unit cell is 8 x 1/8 = 1 atom.
- In body centred cubic unit cells, the number of atoms = 8 x 1/8 + 1 x 1= 2 atoms.
- In face centred cubic unit cell, the number of atoms = 8 x 1/8 + 6 x 1/2 = 4 atoms.
Density of a Unit Cell:
Let us suppose that edge of a unit cell of cubic crystal determined by X-ray diffraction is ‘a‘. Let ‘d‘ is the density of the solid substance and ‘M’ the molar mass, then in case of a cubic crystal,
Volume of a unit cell = a3
Mass of unit cell = Number of atoms in unit cell X mass of each atom = Z X m …………….(i)
Where, Z = number of atoms in one unit cell and m = mass of each atom
But mass of an atom present in the unit cell,
m = M/NA …………..(ii) [NA = Avagadro’s Constant]
Therefore, mass of each unit cell = ZM/NA …………..[Using (ii) in (i)]
Therefore, density of the unit cell, d = Mass of unit cell / Volume of unit cell = Zm/a3 = ZM/NAa3
d = ZM/a3NA
Hence knowing four factors , ‘d‘ can be determined.









Comments (No)